
The Crux of Crystallinity
This page is all about polymer crystals. No, this page has nothing to do with polymers as used by the new age community, using "crystals" to focus psychic energy. We're talking about another kind of crystal here. The kind of crystal we're talking about here is any object in which the molecules are arranged in a regular order and pattern. Ice is a crystal. In ice all the water molecules are arranged in a specific manner. So is table salt, sodium chloride. (Oddly, your mother's good crystal drinking glasses are not crystal at all, as glass is an amorphous solid, that is, a solid in which the molecules have no order or arrangement.)
To understand all this talk of crystals and amorphous solids, it helps to go home. Go home? Why? So you can look in your sock drawer, that's why. You see, some people are very neat and orderly. When they put their socks away they fold them and stack them very neatly. Like this:

Other people don't really care about how neat their sock drawers look. Such folk will just throw their socks in the drawer in one big tangled mess. Their sock drawers look like this:

Polymers are just like socks in that sometimes they are arranged in a neat orderly manner, like the sock drawer in the top picture. When this is the case, we say the polymer is crystalline. Other times there is no order, and the polymer chains just form a big tangled mess, like the socks in the bottom picture. When this happens, we say the polymer is amorphous.
We're going to talk about the neat and orderly crystalline polymers on this page.
So what kind of arrangements do the polymers like to form?
In a perfect part of a perfect world, they would like to line up all stretched out, kind of like a neat pile of new boards down at the lumber yard. Another analogy is uncooked spaghetti right out of the bag like in the picture below.

But they can't always stretch out that straight. In fact, very few polymers can stretch out perfectly straight like that. Instead, all the chain entanglements that they have prevents them from doing so. They look more like the cooked spaghetti below.

There may be some parts like the uncooked noodles above all mixed in like when you don't stir the noodles after you throw them in the pot. Some polymers are especially prone to do this like ultra-high molecular weight polyethylene, and aramids like Kevlar and Nomex. Most polymers can only stretch out for a short distance before they fold back on themselves or get stopped by being all tangled up. You can see this in the picture.
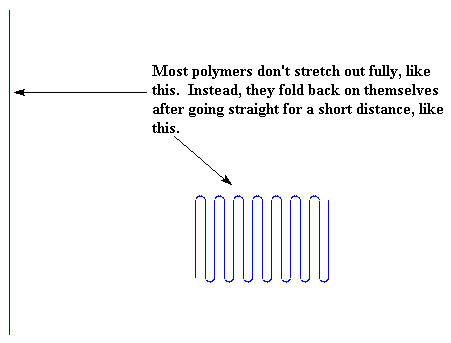
For polyethylene, the length the chains will stretch before they fold is about 100 angstroms.
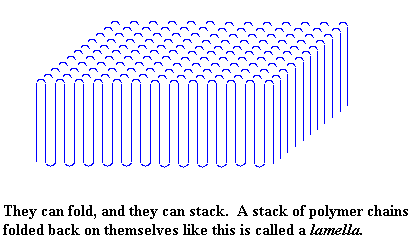
But not only do polymers fold like this, they also form stacks of those folded chains. There is a picture of a bunch of stacks which altogether are called a lamella, right below.
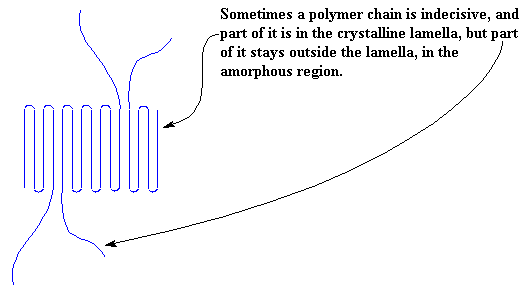
Of course, it isn't always as neat as this. Sometimes part of a chain is included in one crystal, and part of it isn't. When this happens, we get the kind of mess you see below. Our lamella is no longer neat and tidy, but sloppy, with chains hanging out of it everywhere!
Of course, being so mixed up, the polymer chains will often decide they want to come back into the lamella after wandering around outside for awhile. When this happens, we get a picture like this:
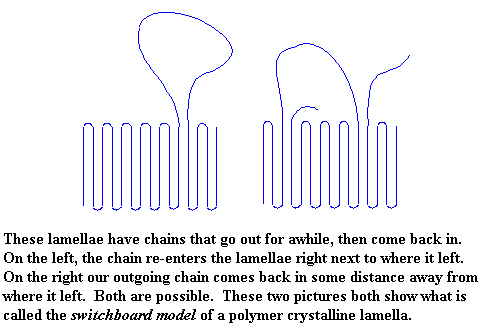
This is the switchboard model of a polymer crystalline lamella. We should tell you that when a polymer chain doesn't wander around outside the crystal, but just folds right back in on itself, like we saw in the first pictures, that is called the adjacent re-entry model. I guess the other kind would just be the messy re-entry model, no?
Amorphousness and Crystallinity
Are you wondering about something, looking at these pictures? Of course, you can see that some of the polymer is crystalline, but then, some is not! Yes folks, most crystalline polymers are not entirely crystalline. In fact, most are mostly not crystalline. The chains, or parts of chains, that aren't in the crystals have no order to the arrangement of their chains. We scientists say that they are in the amorphous state. So a crystalline polymer really has two components: the crystalline portion and the amorphous portion. The crystalline portion is in the lamellae ordered regions, and the amorphous potion is outside the lamellae and between the layers. If we look at a wide-angle picture of what a lamella looks like, we can see how the crystalline and amorphous portions are arranged.
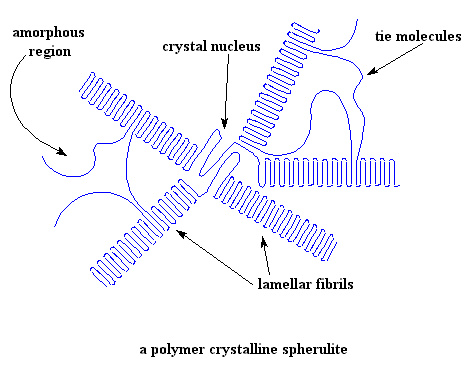
So now you can see, lamella grow like the spokes of a bicycle wheel from a central nucleus. Sometimes we call these spokes "lamellar fibrils". The fibrils grow out in three dimensions, so they really look more like spheres than wheels. The whole assembly is called a spherulite. In a sample of a crystalline polymer weighing only a few grams, there are many billions of spherulites.
In between the crystalline lamellae, there are regions where there is no order to the arrangement of the polymer chains. These disordered regions are the amorphous regions we were talking about.
As you can also see in the picture, a single polymer chain may be partly in a crystalline lamella, and partly in the amorphous state. Some chains even start in one lamella, cross the amorphous region, and then join another lamella. These chains are called tie molecules. They provide a unique property of, you guessed it, tying the lamellae to each other. In a sense, they act like crosslinks and strengthen the solid polymer, giving it better mechanical properties.
The key point here is that no polymer is completely crystalline. If you're making plastics, this is a good thing. Crystallinity makes a material strong, but it also makes it brittle. A completely crystalline polymer would be too brittle to be used as plastic. The amorphous regions give a polymer toughness, that is, the ability to bend without breaking and the ability to absorb impact energy. These are both good properties to have.
However, for making fibers we like our polymers to be as crystalline as possible. This is because a fiber is really a long crystal, in a sense. Want to know more? Then visit the Fiber Page!
Ok, then, most "crystalline" polymers have a mix of amorphous and crystalline regions, but some are more highly crystalline and some are more highly amorphous. Here are some of the polymers that tend toward the extremes:
As you can see in the lists above, there are two kinds of polystyrene. There is atactic polystyrene, and there is syndiotactic
polystyrene. The former is totally amorphous and the later is very crystalline.
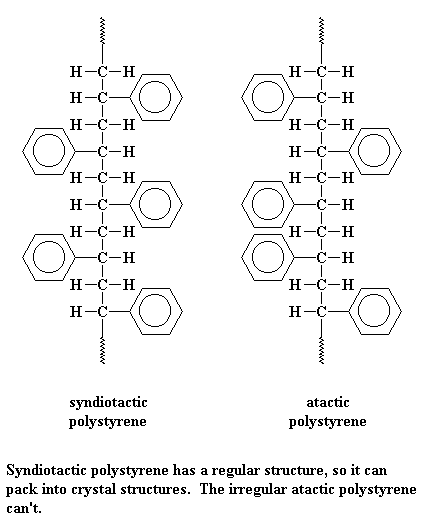
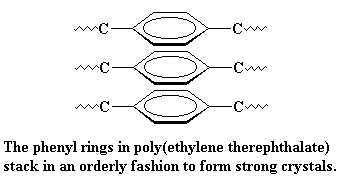
Syndiotactic polystyrene has a very regular and orderly structure, with the phenyl groups falling on alternating sides of the linear carbon backbone. This means it can pack very easily into crystals because the backbones can come close together without the phenyl groups getting in the way. In fact, the phenyl groups are able to also come very close to each other, which they like to do very much. That interaction is called "pi-stacking," and occurs in lots of polymers with aromatic rings in the backbone or pendent to the polymer chains.
But atactic styrene has no such order. The phenyl groups come on any which side of the chain they please going down the backbone. With no order, the chains can't pack very well plus the pi-stacking isn't there as much. So atactic polystyrene is very amorphous. Does that mean it has lousy properties? Of course not! Atactic polystyrene is one of the most widely used commercial polymers in products today. Take a look at the stores in level one and see how many items contain this polymer.
Other atactic polymers like poly(methyl methacrylate) and poly(vinyl chloride) are also amorphous. And as you might expect, stereoregular and symmetrical polymers like isotactic polypropylene and
polytetrafluoroethylene are highly crystalline. But of course, not completely so. That's why these polymers have such incredible combinations of properties, strong AND tough.
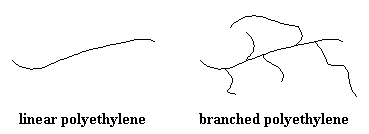
Polyethylene is another good example. It can be crystalline or amorphous. Linear polyethylene can be nearly 100% crystalline, depending on how it's processed and handled. But the branched stuff just can't pack the way the linear stuff can, with the branch points getting in the way, so it is more highly amorphous. It still has some crystalline domains, though, and those act as physical crosslinking sites. Low density polyethylene (highly branched version) is weaker than the other, more linear kinds, but still useful for sandwich bags and food wrap.
Polyesters are another example. Let's look at the polyester we call poly(ethylene terephthalate) or PET.
The polar ester groups make for strong interactions, just like the poles of a magnet pull toward each other. In addition, the aromatic rings like to stack together in an orderly fashion (remember pi-stackin?), making the crystal even stronger. These
strong interaction again raise the Tm of PET much higher than that of polyethylene.
Some Highly Crystalline Polymers:
Some Highly Amorphous Polymers: Polypropylene
Poly(methyl methacrylate) Syndiotactic polystyrene
Atactic polystyrene Nylon
Polycarbonate
Kevlar and Nomex
Polyisoprene
Polyketones
Polybutadiene
Why?
So why is it that some polymers are more highly crystalline and some are more highly amorphous? There are two important factors, polymer structure and intermolecular forces.
Crystallinity and polymer structure
A polymer's molecular structure strongly affects crystallinity. If it's regular and orderly, highly symmetrical, it will pack into crystals more easily. In a way, the molecules want to get next to each other in crystalline domains. If the molecular structure is not highly regular and symmetrical, it won't. Let's take a look at polystyrene in it's various forms to help us understand how this works.
Crystallinity and intermolecular forces
Intermolecular forces can be a big help for a polymer if it wants to form crystals. A good example is nylon. You can see from the picture below that the polar amide groups in the backbone chain of nylon 6,6 are strongly attracted to each other. They form strong intermolecular hydrogen bonds. This strong binding holds chains together, and because those chains are so symmetrical, they're also form crystals. This raises the melting point of the crystals compared to polymers without such strong intermolecular interactions. That's why nylons have much higher melting points than, say, polyethylene or polypropylene.
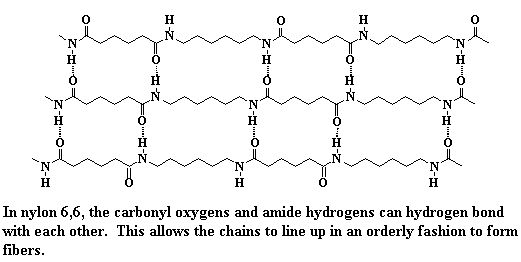
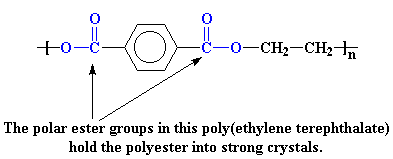
And now you might be asking yourself, "If those intermolecular forces affect crystallinity and Tm, don't they also affect Tg? Wouldn't stronger interactions causing a higher Tm also lead to a higher Tg?"
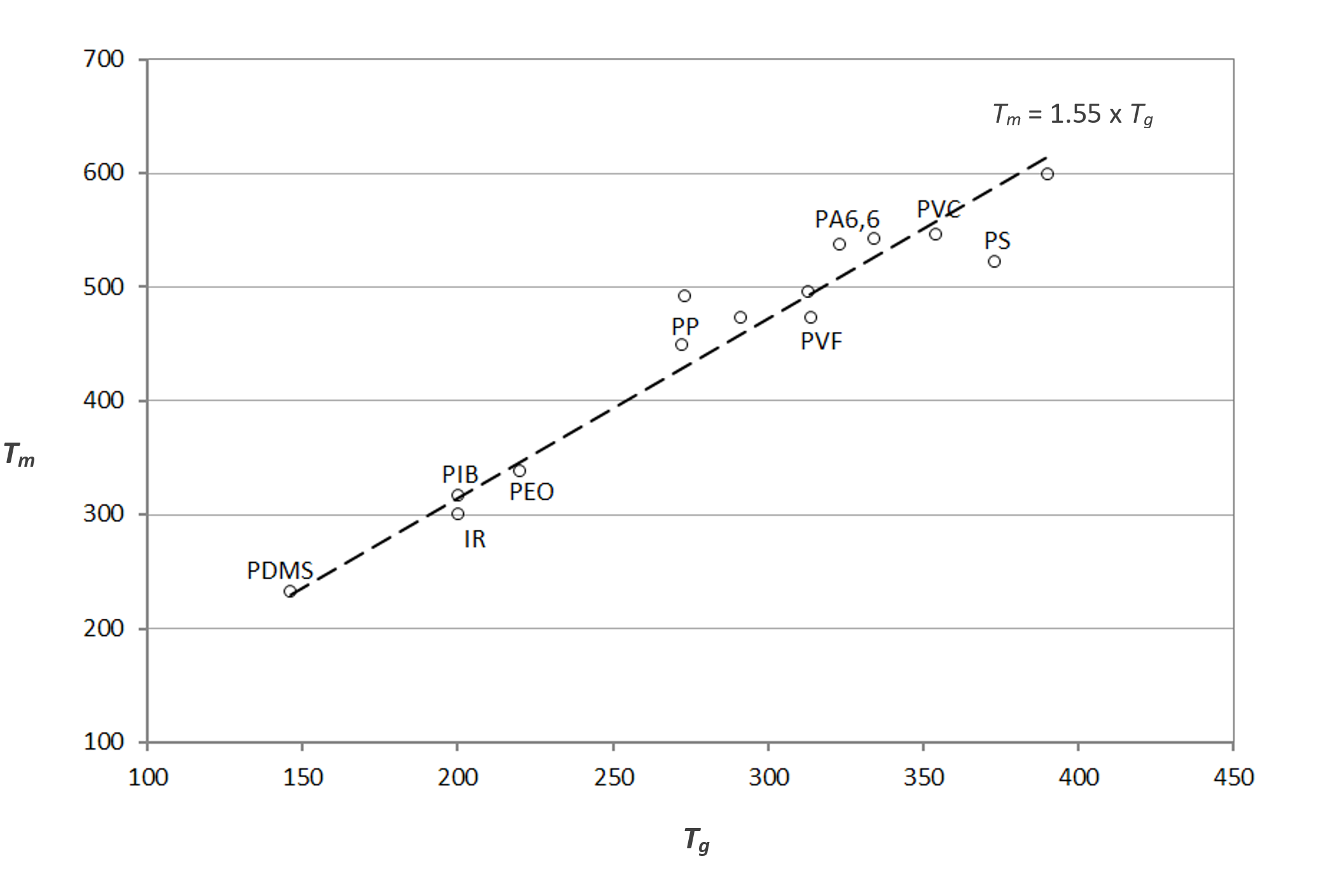
Wow! You've come a long way already. Great questions and the answer to both is "Yes!" In fact, for many polymers, there's a more-or-less linear relationship between Tg and Tm (degrees Kelvin). Take a look at the plot below for some of the more common polymers that are at least semi-crystalline. And as you might have guessed, Tm is always greater than Tg.
And just in case you're wondering about physical properties, here's a brief summary of several properties for a couple dozen common polymers. These include some pretty high perfomance materials as well as some that aren't (like low density PE).
| Acronym | Polymer Name | Tg (oC) | Tm (oC) | TGA* |
Linear CTE** | Flex Modulus | Useful Conversions |
|---|---|---|---|---|---|---|---|
| ABS | Acrylonitrile-Butadiene-Styrene | 110-125 | ----- | 375 | 65-95 | 2.07 - 4.14 | 1 Pa = 10 dynes/cm2 |
| PMMA | Poly(methyl methacrylate) | 85-110 | ----- | 313 | 50-90 | 2.24 - 3.17 | 1 psia = 6.895 kPa |
| AN | Polyacrylonitrile |
95 | 135 | ---- | 66 | 3.45 - 4.07 | 1 kPa = 0.145 psia |
| PTFE | Polytetrafluoroethylene | 126* | 327 | 525 | 70 - 120 | 0,525 | 1 MPa = 1,000,000 Pa |
| PVDF | Poly(vinylidene fluoride) | -60 to -20 | 170 - 178 | 470 | 70 - 142 | 1.72 - 2.89 | 1 GPa = 1,000 MPa |
| Nylon 6 | Polycaprolactam | 40 - 87* | 210 - 220 | 400 | 80 - 83 | 2.69 | 1 Newt = 101.97 G force |
| Nylon 66 | Nylon 66 |
50* | 255-265 | 426 | 80 | 2.83-3.24 | 1 Joule = 0.239 calories |
| PC | Polycarbonate | 140 - 150 | ---- | 473 | 68 | 2.35 | 1 calorie = 4.184 Joules |
| PBT | Poly(butylene terephthalate) |
30 - 60 | 220 - 287 | 386 | 60 - 95 | 2.28 - 2.76 | oF = 9/5(oC) + 32 |
| PET | Poly(ethylene terephthalate) | 73 - 80 | 245 - 265 | 414 | 65 | 2.41 - 2.10 | oC = 5/9(oF - 32) |
| PEEK | Poly(ether ether ketone) | 150 | 334 | 575 | 40 - 108 | 3.86 | 10 Poise = 1 Pa-sec |
| PEI | Poly(ether imide) | 215 - 217 | ---- | ---- | 47 - 56 | 3.31 | |
| LDPE | Low density polyethylene | -133 to -100 | 98 - 115 | 459 | 100 - 220 | 2.40 - 3.30 | |
| HDPE | High density polyethylene | -133 to -123 | 130 - 137 | 469 | 59 - 110 | 1.00 - 1.55 | |
| PI | Polyimide | ---- | 310 - 365 | ---- | 45 - 56 | 3.10 - 3.45 | |
| PPO | Poly(phenylene oxide) | 100 - 142 | ---- | 400 | 38 - 70 | 2.25 - 2.76 | |
| PPS | Poly(phenylene sulfide) | 88 | 285 - 290 | 508 | 49 | 3.79 | |
| PP | Polypropylene |
-15 | 160 - 175 | 417 | 81 - 100 | 1.17 - 1.72 | |
| PS | Polystyrene | 74 - 109 | ---- | 351 | 50 - 83 | 2.62 - 3.38 | |
| PSO | Polysulfone | 190 | ---- | 510 | 56 | 2.69 | |
| PES | Poly(ether sulfone) | 220 - 230 | ---- | ---- | 55 | 2.40 - 2.62 | |
| PVC | Poly(vinyl chloride) | 75 - 105 | ---- | 265 | 50 - 100 | 2.07 - 3.45 |
*data taken from Polymer Handbook, Second Edition, J. Brandup, E.H.Imergut, John Wiley and Sons, New York, NY, 1975
**data from TA Instruments Library (heating rate of 20 oC/min)
How Much Crystallinity?
Remember we said that many polymers contain lots of crystalline material and lots of amorphous material. Almost no polymer is 100% crystalline and, in fact, most polymers are only around 10-30% crystalline. There's a way we can find out how much of a polymer sample is amorphous and how much is crystalline. This method has its own page, and it's called differential scanning calorimetry. It uses an analytical instrument to actually measure the glass transition and melting temperatures. More importantly, it can measure how much of each is in a given sample. This kind of "quantitation" is very important in understanding how well any polymer will behave and what it can be used for. Lesson here is: the more you know, the more you understand.There are other methods that can tell you something about what kind of crystallinity is present, such as neutron and x-ray scattering. Solid state NMR has recently become an important tool for looking at the types and amounts of crystalline and amorphous domains present. We don't have time or space to tell you all about these powerful tools here, but if you're interested, you can find tons of information on the web.
Using a combination of these techniques, it's even possible to differentiate amorphous domains from what's called rigid amorphous areas. The former makes a polymer tougher and more flexible while the latter makes it stronger. Didn't know there were that many different regions inside a perfectly ordinary polymer, did you?
(Spherulite figure after Odian, George; Principles of Polymerization, 3rd ed., John Wiley & Sons, New York, 1991, p.27.)

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |