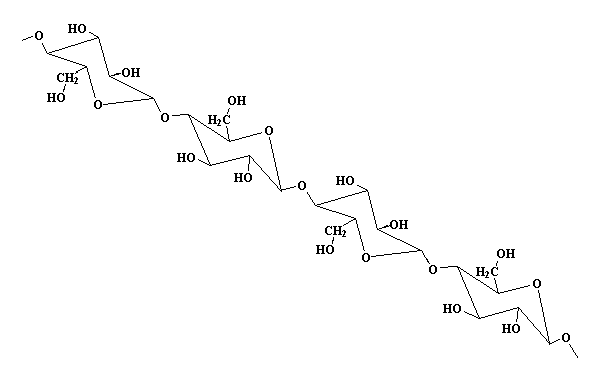
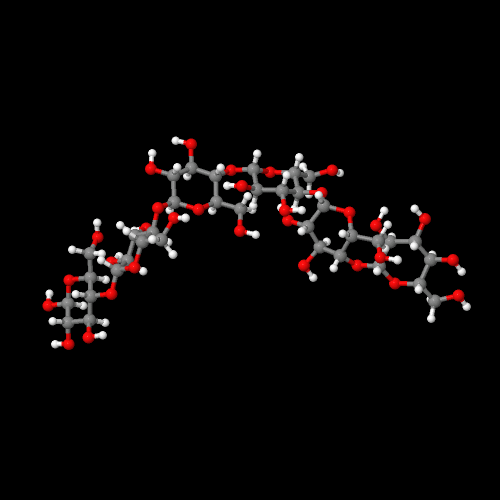
The model above is an image of the pdb model you can view
by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up
with the 3D model in it when you are ready to come back here.
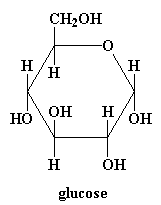
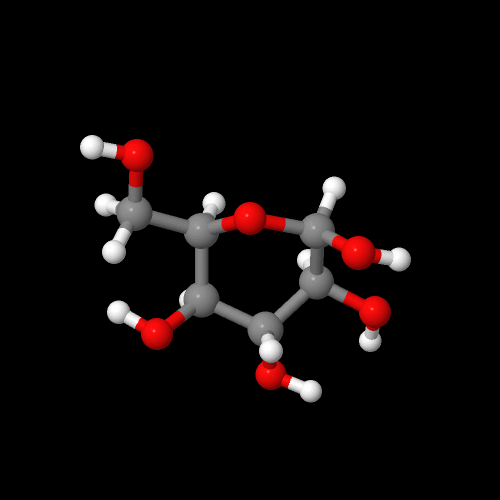
Now take a look at glucose in 3-D! Or click on the model on the right above.
Cellulose has an important place in the story of polymers because it was used to make some of the first synthetic polymers, like cellulose nitrate, cellulose acetate, and rayon. Click here to find out more.
Clean hair
Another cellulose derivative is hydroxyethylcellulose. It differs from plain ol' regular cellulose in that some or all of the hydroxyl groups (shown in red) of the glucose repeat unit have been replaced with hydroxyethyl ether groups (shown in blue).
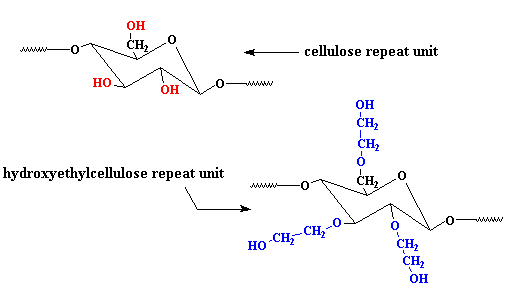
These hydroxyethyl groups get in the way when the polymer tries to crystallize. Because it can't crystallize, hydroxyethylcellulose is soluble in water. In addition to being a great laxative, it's used to thicken shampoos as well. It also make the soap in the shampoo less foamy, and it helps the shampoo clean better by forming colloids around dirt particles.
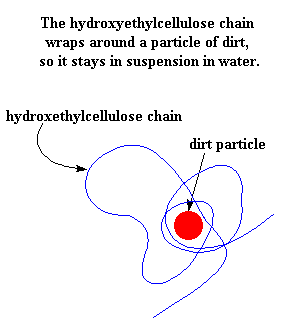
Normally, particles of dirt are insoluble in water. But a chain of hydroxyethylcellulose (shown in blue) can wrap itself around a dirt particle (shown in red). This mass can be thought of as a snack cake, with the polymer chain as the cake and the dirt as the creamy filling. This snack cake is soluble in water, so by wrapping around the dirt like this, the hydroxyethylcellulose tricks the water into accepting the dirt. In this way, the dirt gets washed away instead of being deposited back onto your hair.
Visit another polysaccharide
A polysaccharide that's very similar to cellulose is starch. To find out just how starch and cellulose are different, click here.Other polymers that are used as fibers include:

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |
