
Noshing on
Natural Polymers
Years ago, before there were plastics and synthetic polymers, in fact, all the way back to the beginning of life on earth, nature was using natural polymers to make life possible. We don't think of natural polymers in the same way as synthetic polymers because we can't take credit for them as marvels of our own ingenuity. And while chemical companies can't sell most of them for profit, many natural polymers are incredibly useful. Where would be without edible versions such as starch, protein and collagen? No where, that's exactly where!
Natural polymers include the RNA and DNA that are so important in genes and life processes. In fact, messenger RNA is what makes possible proteins, peptides, and enzymes. Enzymes help do the chemistry inside living organisms and peptides make up some of the more interesting structural components of skin, hair, and even the horns of rhinos. Other natural polymers include polysaccharides (sugar polymers) and polypeptides like silk, keratin, and hair. Natural rubber is, naturally a natural polymer also, made from just carbon and hydrogen. Let's look at each of the main families of natural polymers closely.
Polysaccharides
DNA and RNA
RNA and DNA contains polymer backbones which are based on sugar units. This makes them polysaccharides, although in the case of RNA and DNA, there are well ordered groups attached to the sugar units that give these polymers their unique capabilities.Wood and Potatoes
 Another family of polysaccharides includes starch and cellulose.
Starch
is a high molecular weight polysaccharide. Foods like bread, corn, and
potatoes are full of starch. Starch may
have as many as 10,000 sugar units all linked together. The way these
units link up, all in a linear arrangement or with some of them
forming branches, determines what kind of starch or polysaccharide
it is (more about this later). Another very important member
of the polysaccharide family is cellulose.
This is the main polymer that makes up plants and trees. Wood is
primarily cellulose This
polymer is different than starch. (Click here
to find out more.) Starch is soluble n hot water and can easily
be made into useful objects. Cellulose, on the other hand, is
highly crystalline
and almost totally insoluble in anything. Cotton is a form of
cellulose that we use in most of our clothes. The fact that it
is insoluble in hot water is important. Were it otherwise our
clothes would dissolve when we washed them. Cellulose also has
the neat property that when you wet it and run a hot iron over
it, it smoothes out and flattens down. This makes our cotton
clothes look nice (at least for a little while) but still allows
them to clean up easily when we wash them.
Another family of polysaccharides includes starch and cellulose.
Starch
is a high molecular weight polysaccharide. Foods like bread, corn, and
potatoes are full of starch. Starch may
have as many as 10,000 sugar units all linked together. The way these
units link up, all in a linear arrangement or with some of them
forming branches, determines what kind of starch or polysaccharide
it is (more about this later). Another very important member
of the polysaccharide family is cellulose.
This is the main polymer that makes up plants and trees. Wood is
primarily cellulose This
polymer is different than starch. (Click here
to find out more.) Starch is soluble n hot water and can easily
be made into useful objects. Cellulose, on the other hand, is
highly crystalline
and almost totally insoluble in anything. Cotton is a form of
cellulose that we use in most of our clothes. The fact that it
is insoluble in hot water is important. Were it otherwise our
clothes would dissolve when we washed them. Cellulose also has
the neat property that when you wet it and run a hot iron over
it, it smoothes out and flattens down. This makes our cotton
clothes look nice (at least for a little while) but still allows
them to clean up easily when we wash them.
Chitin: The Polymer for the Seafood Lover in You!
 Another member of the polysaccharides is chitin. It makes up the shells
of crawfish, shrimp, crabs, lobsters, and other crustaceans. It is hard,
insoluble…and yet
somehow flexible. We haven't figured out how to make synthetic polymers
that have this neat combination of properties. We also haven't
figured out how to do much with chitin, although we do use cellulose
for a lot of chemical applications and to make paper, wooden
houses, wooden shoes, and the like. There's a lot of research
underway to use chitin for different stuff, and maybe someday
we'll make clothes or plastic out of it. This is an area of research
that its important since it uses natural polymers that come from
renewable resources or waste products. (Do you know how many
shrimp lose their shells every year for us?)
Another member of the polysaccharides is chitin. It makes up the shells
of crawfish, shrimp, crabs, lobsters, and other crustaceans. It is hard,
insoluble…and yet
somehow flexible. We haven't figured out how to make synthetic polymers
that have this neat combination of properties. We also haven't
figured out how to do much with chitin, although we do use cellulose
for a lot of chemical applications and to make paper, wooden
houses, wooden shoes, and the like. There's a lot of research
underway to use chitin for different stuff, and maybe someday
we'll make clothes or plastic out of it. This is an area of research
that its important since it uses natural polymers that come from
renewable resources or waste products. (Do you know how many
shrimp lose their shells every year for us?)
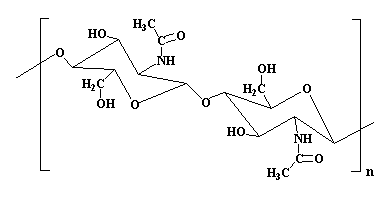
Chemically, chitin is poly(N-acetylglucosamine). Here's it's structure:
We Learn from Nature
As you take a look more closely at each member of these families of natural polymers, remember this: nature was there first, by a long shot! One of our jobs as scientists is to figure out how nature does such a good job so that we can imitate it. For example, once we figured why silk had such neat properties we were able to make synthetic silk in the form of nylons. We still have a long way to go, though, before we can make synthetic RNA and DNA that will lead to synthetic life. While we may never get there, trying to figure out how is fun and leads to lots of important developments in synthetic polymers and other areas including medicine and biochemistry. This raises the important point that science is like life. It doesn't deal with just one thing, but everything mixed together. Polymer science isn't the only science, and it may not even be the most important science (although we in the business like to think it is!). It is one of the areas that can help us understand and use the knowledge we get from studying nature. In this way, we develop technology.
(Note: Just to clear up this whole science-and-technology question, science and technology are two different things despite being so closely related. Science is the act of gathering knowledge by observation and experimentation. Technology is putting this knowledge to use. Example: Using science we learn that hot gasses expand. Then using technology, we use the principle hot gasses expanding to make a gasoline engine that can power a car. See how it works?)
Proteins and Polypeptides
Proteins
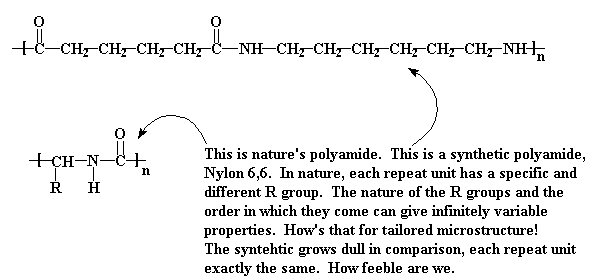
Proteins were the first examples of polyamides (a fancy word for nylon). Both share many common traits but they are very different in how they are made and in their physical properties. They are alike in that the both contain amide linkages in the backbone. Amides are made from carboxylic acid groups and amine groups through the loss of water. (For more on this click here.)The amide molecular segment is unique in its structure and intermolecular interactions. Because of the hybridization of the nitrogen, carbon, and oxygen of the amide group, the segment is basically flat. More importantly, the hydrogen on the nitrogen and the carbonyl oxygen are capable of a strong interaction called a hydrogen bond. Because of this, the amide groups like each other so much that they form strong associations that give amide-containing polymers unusual properties. This type of interaction is also discussed in the section on nylons, and is the key similarity between natural and synthetic polyamides.
The differences between how nature does nylons and how we do it is striking. We mostly make nylons from molecules that have lots of CH2 groups in them. The section on nylons shows structures for nylon 6 and nylon 6,6, two of the most common synthetic polyamides. They possess four, five, or six CH2 groups between amide units. Nature, however, is much more economical, choosing to use only a single carbon between amide groups. What nature does differently is to substitute this carbon with lots of different functional segments and groups.
This results in two key properties. First, the individual segments and the entire molecule are optically active, or chiral. This means they are like gloves: there are both right and left and versions. For some reason, nature chose to use only the left hand version of the amino acids that are synthesized by plants and animals. The fact that only one of the two isomers is used leads to some neat stereochemical consequences. For example, natural polypeptides can form helical structures while nylons can't. The helical conformations increase the stability of the natural polypeptides. Did you know that some bacteria can survive in boiling water? This is because their natural polymers have been stabilized by such helical structures. The figure below shows one such helical structure, called an a-helix. Small segments of such helical structures are what nature uses to mold enzymes into certain shapes so that they can do their catalytic magic. For example, a flexible randomly coiled segment may be joined by two a-helix segments so that they can react together on some substrate.
Enzymes
Enzymes are one of the key types of polypeptides and are crucial to life on earth. All living organisms use enzymes to make, modify, and chop up the polymers discussed here. Enzymes are the catalysts that do specific jobs. In fact, oftentimes each enzyme does only one type of job or makes only one kind of molecule. This means that there have to be lots of different enzymes, all made of different combinations of amino acids joined in unique ways in polypeptides, to do all the jobs that any living organism needs done. We know that every creature on earth has hundreds or even thousands of different enzymes to do all the jobs that it requires. What's really strange is that each one of the enzymes has to be made by other enzymes. This leads to very complicated control mechanisms: we don't have the faintest idea (in most cases) how nature decides what enzymes need to be made and when, or how the enzymes are turned on and off. We are beginning to figure this out, and the study of such systems is an important part of biochemistry and biology.
Riding Down the Silk Road
One of the unique polypeptides that we used very early for its superb properties was silk. Silk was discovered by the Chinese long before the birth of Christ. Silk is made by tiny caterpillars trying to spin cocoons for their transformation into moths. We steal the silk from the caterpillars which leaves them in limbo, pretty much. The silk is spun into fibers.Bundles of very thin individual polymers joined together to be stronger. This is the way we also make rope, using weak individual strands bound together in such a way that the total is both flexible and strong. The structure of silk molecules is unusual for a polypeptide. It possesses lots of the unsubstituted amino acid, glycine. Glycine segments are able to form flat extended chains that can pack together nice and tightly. This gives silk its unique strength and shiny flexibility.
Silk has such unique properties, especially in hot wet climates, that it dominated trade for centuries in east Asia. The silk trade between Japan and China controlled the economics of civilizations in that region for longer than either country cares to admit. Even in America, silk was important before World War II for use in silk stockings. When silk was used for parachute cord, women in America got very upset. This resulted in chemical companies synthesizing artificial silk, nylon, to make nylon stockings so that women's feet could stay warm and the men could go back to fighting their wars.
Another key difference between polypeptides and nylons is the way they are made. We humans make nylons in tons per day in huge chemical plants where simple molecules are joined together in large quantities to give products that we need or want. Nature is much more careful and concise in how she does things. For a living organism to make an enzyme, another enzyme or active species must be involved. The synthesis always involves a template, or recording, of how the individual amino acids are to be joined together to give the final polymer. This template, or map is a messenger RNA (mRNA). The message it carries, of course, is how the peptide-making enzyme involved should make the polypeptide.
Each amino acid is brought to the enzyme by a carrier molecule and is activated for incorporation by a whole cascade family of reaction steps. The enzyme adds a single amino acid, one at a time, as indicated by the mRNA. This is a slow and tedious process and takes a long time. Sometimes the enzyme gets frustrated, waiting for the right amino acid to come along, and slaps a wrong one on instead. To compensate this the enzyme is made to back up occasionally to check its work. If it has made a mistake, it has a process for clipping out the wrong amino acid and inserting the right one. We humans never do this. If we make a mistake, we simply grind it up and throw it away.
Our Feeble Capabilities
We are beginning to understand how nature puts these molecules together and we've figured out how to do it ourselves. However, we're not very good at it and can't make very large molecules vary efficiently. The reason is, if we make a mistake, it ruins the whole molecule and we don't know how to fix it. The kind of machine that's used to make synthetic analogs of polypeptides is called a "peptide synthesizer". Such a machine is built around tiny polymer beads that we attach to the first amino acid which we want to put in our polypeptide chain. (This is called the Merrifield Synthesis Approach. The use of polymer beads was a great creative idea, and Robert Merrifield won the Nobel Prize for it back in 1984.) We than take an activated amino acid, similar to what nature uses, and attach it by forming an amide bond. We repeat this process over and over again, trying to get this reaction to go every time on every molecule on every bead. Sometimes, we are not so lucky and we miss and amino acid. This means that some of the polypeptides have units missing. This always leads to mixtures of good product and bad product, in which the good product may be the minor component. This gets worse the bigger the polypeptide we are trying to make, and is one of the major problems we have with how we make polypeptides. If we could just figure out how to back up and check each one of our additions, and then correct the mistakes that are there, maybe we could do as good a job as nature. Maybe.You're probably asking why we would want to do such a bad job of making synthetic analogs of what nature does so well. (If you aren't, just play along anyway.) There are lots of reasons, one of which is to figure out just how nature does it. Another is to figure out why peptides and enzymes work the way that they do. It's not always clear to us mere mortals why a given sequence of amino acids causes a polypeptide to assume a certain shape or structure. These structures are key to how the polypeptides do whatever job nature has devised for them. Sometimes, when we see the way nature puts these molecules together, we can make synthetic analogs that do the same thing but are easier to make. This has led to the development of new drugs and to treatments for some genetic diseases.
Nature also does things differently than us by synthesizing polypeptides in water. Most of our syntheses, in fact, don't use water. We synthesize our polyamides in toxic organic solvents. This leads us to a problem: what do we do with the organic solvents when we're through? Sometimes we burn the, but more and more we try to recycle these materials. Not only are they getting more expensive to buy in the first place (compared to cheap water which is everywhere, or almost everywhere) but we must be responsible for their recycle, purification, and final disposal. An example of how nature uses water in this way, and one which we still haven't figured out is the production of spider silk. Spiders spin their webs from solutions of polypeptides in water. These solutions are squeezed through the spider's tiny spinneret and elongated quickly to form the spider webs which we've all seen and sometimes gotten tangled in. What's really weird is that, once these spider webs form, they are no longer soluble in water. If we could just figure out how spiders first make spider silk in water and then spin their webs from it, we could make nylon the same way. This might save us a lot of waste disposal problems, and money. This is one area of basic research where we need lots and lots of help; maybe you can think of something we could try.

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |