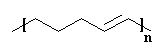What Is Olefin Metathesis?
In these pages we've talked an awful lot about making polymers from monomers with carbon-carbon double bonds. Remember all our talk of vinyl polymers? But vinyl polymers don't have double bonds in the back bone chains. Now we're going to talk about taking monomers with double bonds, and using them to make polymers with double bonds in the backbone chain. We call these polymers polyalkenamers. One way to make polyalkenamers to use a nifty reaction called olefin metathesis.
Olefin is an old word, kind of like betwixt or forsooth. An olefin is the same thing as an alkene, that is a molecule with a carbon-carbon double bond. Olefin metathesis is of course a reaction involving olefins. Two olefins, to be exact. And here's what they do: the double bond carbons change partners, to form two new olefins, just like in the little picture here:
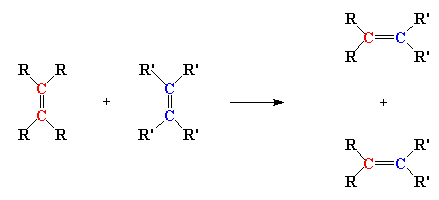
Neato, huh? But it isn't really obvious that this reaction can be used to make polymers. In fact, this reaction was discovered in the 1920s, and it wasn't until about fifty years later that scientists figured out a way to make polymers out of it. And it wasn't until around twenty years later still that anyone made anything useful with the reaction. So turning this reaction into a polymerization took some clever scheming on the part of some polymer chemists. But succeed they did, and in fact, they came up with two ways of using olefin metathesis to make polymers.
TWO GREAT POLYMERIZATIONS,
ONE LOW PRICE!
So let's take a look at them why don't we?
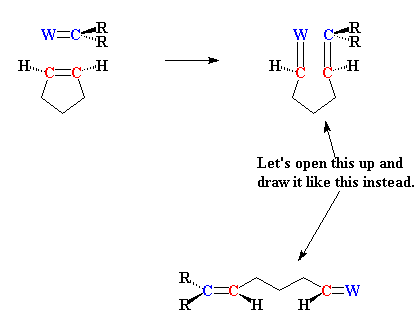
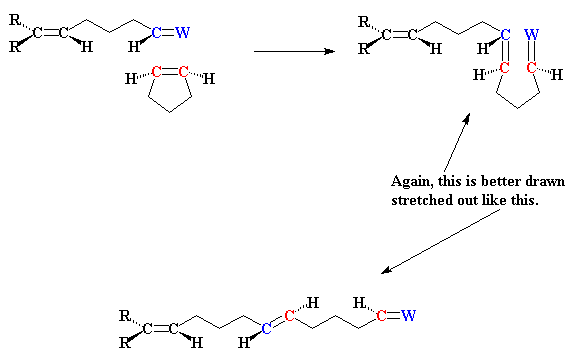
How does metathesis make this possible? I'll show you how, using the
example of two molecules of 1,5-hexadiene reacting together:
And of course the double bonds at the ends of the new molecule can have
a little metathesis magic done on them as well, and in this way the
polymer grows. I showed the cis- isomer of the product formed,
but usually both cis- and trans- isomers can form, and
there's usually room for both in the polymer that we end up with.
We can get polymers from cyclic olefins, too. Look at the picture below,
and you see that a cyclic olefin, cyclopentene in this case, is used to
make a polymer which doesn't have cyclic structures in its backbone.
This is why we call it "ring-opening metathesis polymerization". Makes
sense. ROMP is, believe it or not, used to make some useful products.
A nifty little molecule, norbornene, is polymerized by ROMP to get
polynorbornene. It's a kind of rubber used to make auto parts, the
little body-mount parts that keep things from vibrating and such.
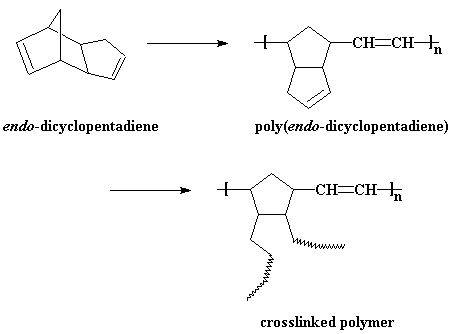
We can also make a polymer from a similar molecule using ROMP. That
molecule is endo-dicyclopentadiene. When we polymerize it we get
a polymer with a cyclic olefin in a pendant group. This polymer is polydicyclopentadiene, of course.
This pendant olefin can be reacted to crosslink the
polymer,
probably by traditional vinyl
polymerization of some sort. The polymer is a hard plastic that is
used to make satellite dish antennae, body parts for snowmobiles, and
other things that are generally used outside. You don't want to use this
stuff inside, because there's always a little bit of
endo-dicyclopentadiene that hasn't reacted left trapped in the
polymer. This is one malodorous monomer, and it doesn't take much to
stink up a room.
How this happens takes some explaining. It's kind of
complicated. It starts off with an initiation step.
Ok, kids what do we need for an initiation step? Maybe, an initiator?
The initiator in this case is usually a compound with a carbon atom
double bonded to a metal atom, like tungsten or molybdenum. The catalysts
often look like this, but we're going to simplify the drawing.
So the initiator reacts with a molecule of cyclopentene in a metathesis
reaction like we've been seeing, to get a molecule with a carbon-carbon
double bond at one end, and a carbon-metal double bond at the other.
That carbon-metal double bond can react with another molecule of cyclopentene in the exact same way:
And in this way the polymer grows, until we get this:
Metathesis Polymerization #1:
The two different polymerizations
are called ring opening metathesis polymerization, or ROMP, and acyclic diene metathesis
polymerization, or ADMET. We'll talk about ADMET first because it's simpler,
even though ROMP has been more thoroughly researched. In ADMET we start with
an acyclic diene, surprise, surprise, such as 1,5-hexadiene, and end up
with a polymer with a
double bond in the backbone chain, plus by-product ethylene gas.
Acyclic Diene Metathesis Polymerization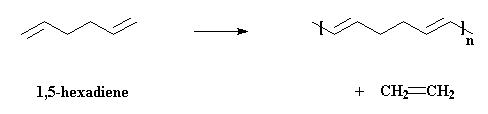
Metathesis Polymerization #2:
Ring Opening Metathesis Polymerization
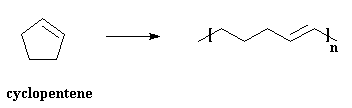
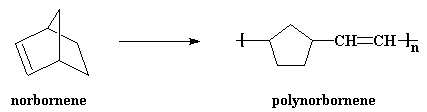
Click here!