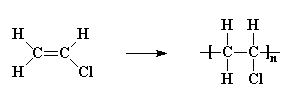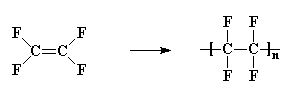
Vinyl polymers are polymers made from vinyl monomers;
that is, small molecules containing carbon-carbon double bonds.
They make up largest family of polymers. Let's see how we get
from a vinyl monomer to a vinyl polymer using for an example the
simplest vinyl polymer, polyethylene.
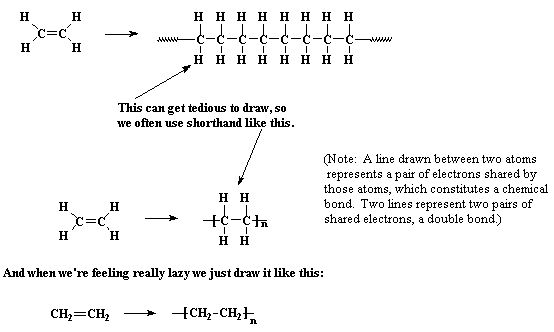
Polyethylene is made from the monomer ethylene, which is also
called ethene. When polymerized, the ethylene molecules are joined
along the axes of their double bonds, to form a long chain of
many thousands of carbon atoms containing only single bonds between atoms.
More sophisticated vinyl polymers are made from monomers in which
one or more of the hydrogen atoms of ethylene has been replaced
by another atom or groups of atoms. Let's see what we can do
by replacing just one of those hydrogen atoms. We can get a number of
common plastics:
polypropylene
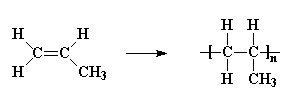
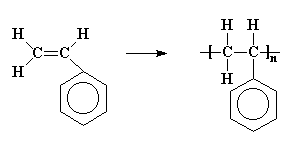
Polystyrene
poly(vinyl chloride)
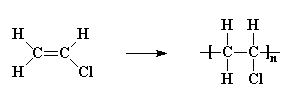
Replacing two hydrogen atoms, on the same carbon atom, we can
get
polyisobutylene, which is type of rubber.
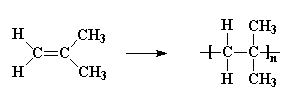
poly(methyl methacrylate)
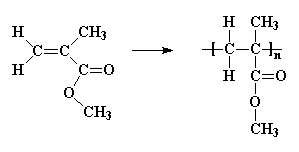
Not many monomers in which hydrogen atoms have been replaced
on both carbon atoms will polymerize. But one polymer that is made from
a monomer substituted on both carbon atoms is polytetrafluoroethylene,
which DuPont makes and calls Teflon.
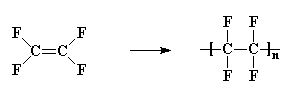
Vinyl polymers are made from vinyl monomers in a variety of ways,
like: