 Polyesters are the polymers, in the form of fibers, that were used back in the seventies
to make all that wonderful disco clothing, the kind you see being modeled
on the right. But since then, the nations of
the world have striven to develop more tasteful uses for polyesters, like
those
nifty shatterproof plastic bottles that hold your favorite refreshing
beverages, like the blue bottle in the picture below. So you
see, polyesters can be both plastics and fibers. Another place you find polyester is in
balloons. Not the cheap ones that you use for water balloons, those are
made of natural rubber. I'm talking about
the fancy ones you get when you're in the hospital. These are made of
a polyester film made by DuPont called Mylar. The balloons are made of a
sandwich, composed of Mylar and aluminum foil.
Materials like this, made
of two kinds of material, are called composites.
Polyesters are the polymers, in the form of fibers, that were used back in the seventies
to make all that wonderful disco clothing, the kind you see being modeled
on the right. But since then, the nations of
the world have striven to develop more tasteful uses for polyesters, like
those
nifty shatterproof plastic bottles that hold your favorite refreshing
beverages, like the blue bottle in the picture below. So you
see, polyesters can be both plastics and fibers. Another place you find polyester is in
balloons. Not the cheap ones that you use for water balloons, those are
made of natural rubber. I'm talking about
the fancy ones you get when you're in the hospital. These are made of
a polyester film made by DuPont called Mylar. The balloons are made of a
sandwich, composed of Mylar and aluminum foil.
Materials like this, made
of two kinds of material, are called composites.
 A special family of polyesters are polycarbonates.
A special family of polyesters are polycarbonates.
Polyesters have
hydrocarbon backbones which contain ester linkages, hence the name.
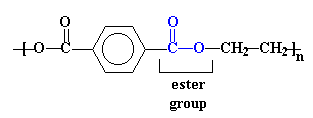
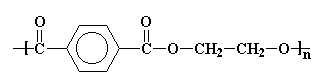
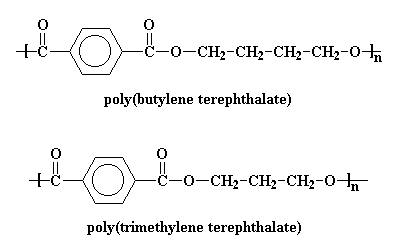
The structure in the picture is called poly(ethylene terephthalate), or
PET for short, because it is made
up of ethylene groups and terephthalate groups (duh!). I realize that
terephthalate is not the kind of word most English-speaking mouths
are used to saying, but with practice you should be able to say it with
only a slight feeling of awkwardness when it rolls off your tongue.
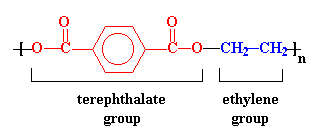
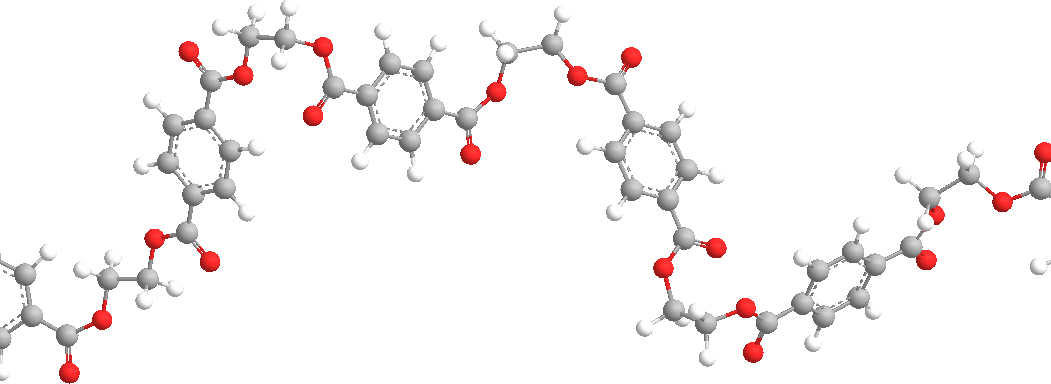
The ester groups in the polyester chain are polar, with the carbonyl oxygen atom having a somewhat negative charge and the carbonyl carbon atom having a somewhat positive charge. The positive and negative charges of different ester groups are attracted to each other. This allows the ester groups of nearby chains to line up with each other in crystal form, which is why they can form strong fibers.
The inventor who first discovered how to make bottles from PET was Nathaniel Wyeth. He's the brother of Andrew Wyeth the famous painter. But others had tried before. Go read this story of someone who may have been the first person to try to make a shatterproof bottle.
Now I'm sure everyone out there is just dying to have two questions
answered. The first one is:
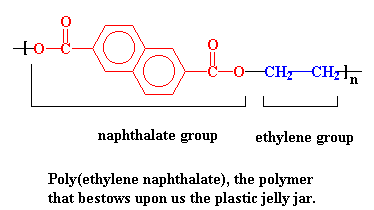
PEN has a higher glass transition temperature than PET. That's the temperature at which a polymer gets soft. The glass transition temperature of PEN is high enough so that it can withstand the heat of both sterilizing bottle washing and hot strawberry jelly. PEN is so good at standing the heat that you don't even have to make the bottle entirely out of it. Just mixing some PEN in with the old PET gives a bottle that can take the heat a lot better than plain old PET.
Ok here goes...
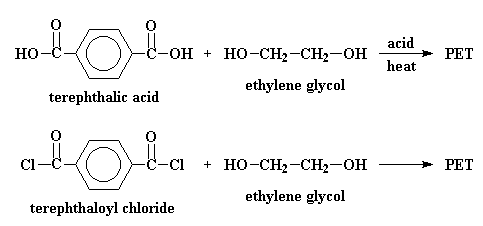
In the big plants where they make polyester, it's normal to start off with a compound called dimethyl terephthalate. This is reacted with ethylene glycol is a reaction called transesterification. The result is bis-(2-hydroxyethyl)terephthalate and methanol. But if we heat the reaction to around 210 oC the methanol will boil away and we don't have to worry about it anymore.
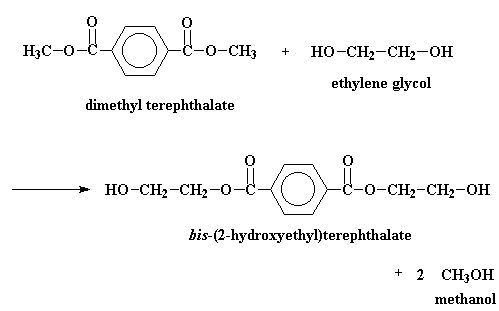
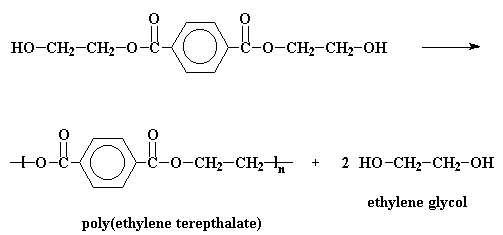
But in the laboratory, PET is made by other reactions. Terephthalic acid and ethylene glycol can polymerize to make PET when you heat them with an acid catalyst. It's possible to make PET from terephthoyl chloride and ethylene glycol. This reaction is easier, but terephthoyl chloride is more expensive than terephthalic acid, and it's a lot more dangerous.
Other polymers used as plastics:| |
Other polymers used as fibers: | |
| Polypropylene | Polypropylene | |
| Polyethylene | Polyethylene | |
| Polystyrene | Nylon | |
| Polycarbonate | Kevlar and Nomex | |
| PVC | Polyacrylonitrile | |
| Nylon | Cellulose | |
| Poly(methyl methacrylate) | Polyurethanes |

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |