
Keywords
amorphous,
crystal,
elastomer,
thermoplastic
We have to make something clear at this point. The glass transition is not the same thing as melting. Melting is a transition which occurs in crystalline polymers. Melting happens when the polymer chains fall out of their crystal structures, and become a disordered liquid. The glass transition is a transition which happens to amorphous polymers; that is, polymers whose chains are not arranged in ordered crystals, but are just strewn around in any old fashion, even though they are in the solid state.
But even crystalline polymers will have a some amorphous portion. This portion usually makes up 40-70% of the polymer sample. This is why the same sample of a polymer can have both a glass transition temperature and a melting temperature. But you should know that the amorphous portion undergoes the glass transition only, and the crystalline portion undergoes melting only.
Now, to understand just why polymers with no order to them are hard and brittle below a certain temperature and soft and pliable above it, it can help to think of a polymer in the amorphous state as a big room full of slithering snakes. Each snake is a polymer chain. Now as you may remember, snakes are cold blooded animals, so all their body heat has to come from their surroundings. When it's warm, the snakes are happy, and can go on about their business of slithering and sliding with no trouble at all. They will move all about randomly, over and around each other, and they slither hither and thither, just having a great time, or as good a time as snakes ever have.
But when it gets cold, snakes don't move too much. They slow down without any heat, and tend to just sit still. Now they're still all wrapped around, over, and under each other, but as far as motion is concerned, it just doesn't happen.
Now imagine trying to drive a bulldozer through this room full of snakes. If it's warm, and the snakes are moving, they can quickly slither out of your way, and the bulldozer moves through the room, causing a minimal amount of snake damage. But if it's cold, one of two things will happen to the motionless snakes. Either (A) the snakes will be stronger than the bulldozer, and the bulldozer won't get through, and the snakes will stay put; or (B) the bulldozer will be stronger than the snakes, and they'll get squashed, still not moving anywhere.
Polymers are the same way. When the temperature is warm, the polymer chains can move around easily. So, when you take a piece of the polymer and bend it, the molecules, being in motion already, have no trouble moving into new positions to relieve the stress you have placed on them. But if you try to bend sample of a polymer below its Tg, the polymer chains won't be able to move into new positions to relieve the stress which you have placed on them. So just like in the example of a room full of cold snakes, one of two things will happen. Either (A) the chains are strong enough to resist the force you apply, and the sample won't bend; or (B) the force you apply will be too much for the motionless polymer chains to resist, and being unable to move around to relieve the stress, the polymer sample will break or shatter in your hands.
This change in mobility with temperature happens because the phenomenon we call "heat" is really a form of kinetic energy; that is, the energy of objects in motion. It is actually an effect of random motion of molecules, whether they are polymer molecules or small molecules. Things are "hot" when their molecules have lots of kinetic energy and move around very fast. Things are "cold" when their molecules lack kinetic energy and move around slowly, or not at all.
Now the exact temperature at which the polymer chains undergo this big change in mobility depends on the structure of the polymer. To see how a small change in structure can mean a big change in Tg, take a look at the difference between poly(methyl acrylate) and poly(methyl methacrylate) on the acrylate page.
There is a difference between polymers and snakes that we probably should discuss at this point. An individual snake is not only wiggling around, but actually moving from one side of the room to the other. This is called translational motion. When you walk down the street, presuming you're not like most Americans who never walk anywhere, you are undergoing translational motion. While polymers are not incapable of such motion, mostly they are not undergoing translational motion. But they are still moving around, wiggling this way and that, much like little kids in church. To be sure, by the time we get down to the glass transition temperature, it is already too cold for the polymer molecules, tangled up in each other as they are, to move any distance in one direction. The motion that allows a polymer above its glass transition temperature to be pliable is not usually translational motion, but what is known in the business as long-range segmental motion. While the polymer chain as a whole may not be going anywhere, segments of the chain can wiggle around, swing to and fro, and turn like a giant corkscrew. The polymer samples may be thought of as a crowd of people on a dance floor. While each whole body tends to stay in the same spot, various arms, legs, and whatnot are changing position a lot. When the temperature drops below the Tg, for polymers the party's over, and the long-range segmental motion grinds to a halt. When this long-range motion ceases, the glass transition occurs, and the polymer changes from being soft and pliable to being hard and brittle.
Want to have some fun? First, get your teacher to bring some liquid nitrogen to class. Then put some in a styrofoam cup, and drop in some household objects made from polymers, like rubber bands or plastic wrap. The liquid nitrogen, being nippy as it is, will cool the objects below their glass transition temperatures. Try to bend your rubber band (hold it with a pair of pliers, because you could get frostbite if you try to touch it with your fingers) and it will shatter! Neato, huh? The rubber band will shatter because it's below its glass transition temperature.
Want to know more about the wonderful glass transition? Read these little segments!
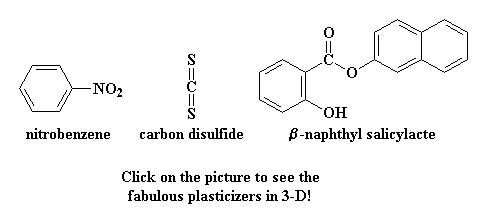
Sometimes, a polymer has a Tg that is higher than we'd like. That's ok, we just put something in it called a plasticizer. This is a small molecule which will get in between the polymer chains, and space them out from each other. We call this increasing the free volume. When this happens they can slide past each other more easily. When they slide past each other more easily, they can move around at lower temperatures than they would without the plasticizer. In this way, the Tg of a polymer can be lowered, to make a polymer more pliable, and easier to work with.
If you're wondering what kind of small molecule we're talking about, here are some that are used as plasticizers:

awww.
Keywords:
It's tempting to think of the glass transition as a kind of melting of the polymer. But this is an inaccurate way of looking at things. There are a lot of important differences between the glass transition and melting. Like I said earlier, melting is something that happens to a crystalline polymer, while the glass transition happens only to polymers in the amorphous state. A given polymer will often have both amorphous and crystalline domains within it, so the same sample can often show a melting point and a Tg. But the chains that melt are not the chains that undergo the glass transition.
There is another big difference between melting and the glass transition. When you heat a crystalline polymer at a constant rate, the temperature will increase at a constant rate. The heat amount of heat required to raise the temperature of one gram of the polymer one degree Celsius is called the heat capacity.
Now the temperature will continue to increase until the polymer reaches its melting point. When this happens, the temperature will hold steady for awhile, even though you're adding heat to the polymer. It will hold steady until the polymer has completely melted. Then the temperature of the polymer will begin to increase once again. The temperature rising stops because melting requires energy. All the energy you add to a crystalline polymer at its melting point goes into melting, and none of it goes into raising the temperature. This heat is called the latent heat of melting. (The word latent means hidden.)
Now once the polymer has melted, the temperature begins to rise again, but now it rises at a slower rate. The molten polymer has a higher heat capacity than the solid crystalline polymer, so it can absorb more heat with a smaller increase in temperature.
So, two things happen when a crystalline polymer melts: It absorbs a certain amount of heat, the latent heat of melting, and it undergoes a change in its heat capacity. Any change brought about by heat, whether it is melting or freezing, or boiling or condensation, which has a change in heat capacity, and a latent heat involved, is called a first order transition.
But when you heat an amorphous polymer to its Tg, something different happens. First you heat it, and the temperature goes up. It goes up at a rate determined by the polymer's heat capacity, just like before. Only something funny happens when you reach the Tg. The temperature doesn't stop rising. There is no latent heat of glass transition. The temperature keeps going up.
But the temperature doesn't go up at the same rate above the Tg as below it. The polymer does undergo an increase in its heat capacity when it undergoes the glass transition. Because the glass transition involves change in heat capacity, but it doesn't involve a latent heat, this transition is called a second order transition.
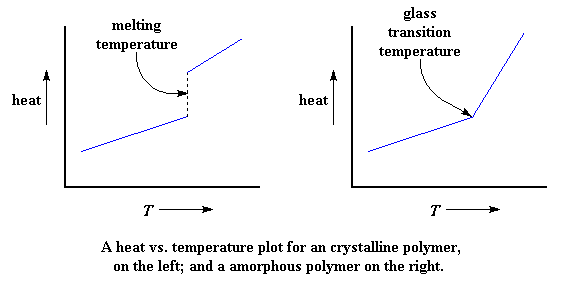
It may help to look at some nifty pictures. The plots show the amount of heat added to the polymer on the y-axis and the temperature that you'd get with a given amount of heat on the x-axis.
The plot on the left shows what happens when you heat a 100% crystalline polymer. You can look at it and see that it's discontinuous. See that break? That's the melting temperature. At that break, a lot of heat is added without any temperature increase at all. That's the latent heat of melting. We see the slope getting steeper on the high side of the break. The slope of this kind of plot is equal to the heat capacity, so this increase in steepness corresponds to our increase in heat capacity above the melting point.
But in the plot on the right, which shows what happens to a 100% amorphous polymer when you heat it, we don't have a break. The only change we see at the glass transition temperature is an increase in the slope, which means, of course, that we have an increase in heat capacity. We can see a heat capacity change at the Tg, but no break, like we do in the plot for the crystalline polymer. As I said before, there is no latent heat involved with the glass transition.
And this, my friends, right before your eyes, is the difference between a first order transition like melting, and a second order transition like the glass transition.
Ok, we know at this point that some polymers have high Tg's, and some have low Tg's. The question we haven't bothered to ask yet is this: why? What makes one polymer glass transition at 100 oC and another at 500 oC?
The very simple answer is this: How easily the chains move. A polymer chain that can move around fairly easily will have a very low Tg, while one that doesn't move so well will have a high one. This makes sense. The more easily a polymer can move, the less heat it takes for the chains to commence wiggling and break out of the rigid glassy state and into the soft rubbery state.
So then I suppose we've brought ourselves to another question...
This is the biggest and most important one to remember. The more flexible the backbone chain is, the better the polymer will move, and the lower its Tg will be. Let's look at some examples. The most dramatic one is that of silicones. Let's take a look at one called polydimethylsiloxane.
This backbone is so flexible that polydimethylsiloxane has a Tg way down at -127 oC! This chain is so flexible that it's a liquid at room temperature, and it's even used to thicken shampoos and conditioners.
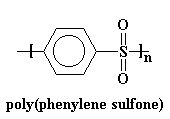
Now we'll look at another extreme, poly(phenylene sulfone).
This polymer's backbone is just plain stiff. It's so rigid that it doesn't have a Tg! You can heat this thing to over 500 oC and it will still stay in the glassy state. It will decompose from all the heat before it lets itself undergo a glass transition! In order to make a polymer that's at all processable we have to put some flexible groups in the backbone chain. Ether groups work nicely.
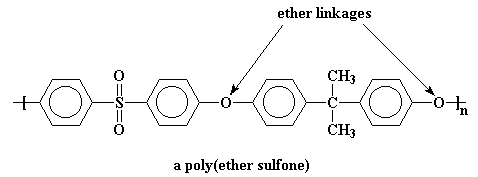
Polymers like this are called poly(ether sulfones), and those flexible ether groups bring the Tg of this one down to a more manageable 190 oC.
Pendant groups have a big effect on chain mobility. Even a small pendant group can act as a fish hook that will catch on any nearby molecule when the polymer chain tries to move like corkscrew. Pendant groups also catch on each other when chains try to slither past each other.
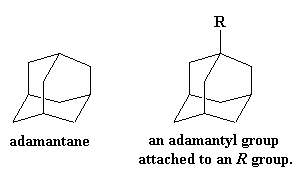
One of the best pendant groups for getting a high Tg is the big bulky adamantyl group. An adamantyl group is derived from a compound called adamantane.
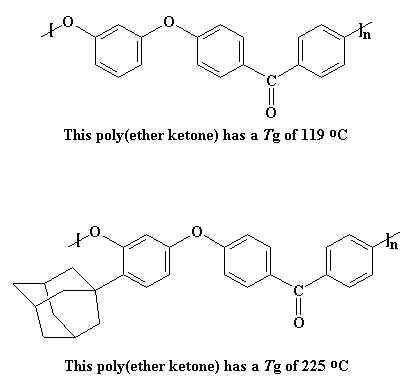
A big group like this does more than just act like a hook that catches on nearby molecules and keeps the polymer from moving. It's a downright boat anchor. Not only does it get caught on nearby polymer chains, its sheer mass is such a load for its polymer chain to move that it makes the polymer chain move much more slowly. To see how much this affects the Tg, just take a look at two poly(ether ketones), one with an adamantane pendant group and one without.
The Tg of the polymer on the top is already decent at 119 oC, but the adamantyl group raises even higher, to 225 oC.
But big bulky pendant groups can lower the Tg, too. You see, the big pendant groups limit how closely the polymer chains can pack together. The further they are from each other, the more easily they can move around. This lowers the Tg, in the same way a plasticizer does. The fancy way to say that there is more room between the polymer chains is to say there is more free volume in the polymer. The more free volume, the lower the Tg generally. We can see this with a series of methacrylate polymers:
You can see a big drop each time we make that pendant alkyl chain one carbon longer. We start out at 120 oC for poly(methyl methacrylate), but by the time we get to poly(butyl methacrylate) the Tg has dropped to only 20oC, pretty close to room temperature.
The Glass Transition vs. Melting
first order transition,
heat capacity,
second order transition
What Becomes the High Tg Polymer?
I'm glad you asked that. There are several things that affect the mobility of a polymer chain. Go look at each one!
Backbone Flexibility
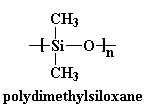
Pendant Groups Part I:
Fish Hooks and Boat Anchors
Click on the adamantane to see it in 3-D!
Pendant Groups Part II:
Elbow Room
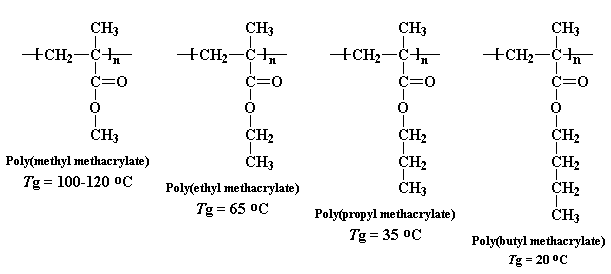

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |