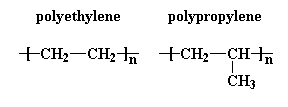

Keywords
amorphous,
copolymer,
entropy,
hydrogen bonding
Polymers Don't MixSometimes we want a material that has the some of the properties of one polymer, and some of the properties of another. Instead of going back into the lab and trying to synthesize a brand new polymer with all the properties we want, we try to mix two polymers together to form a blend, that will hopefully have some properties of both.
Making Polymers Mix
Making Your Own Blends
Properties of Blends
To Blend or Not to Blend
Polymers Don't Mix
Sounds easy enough, but it turns out that blending two different kinds of
polymers can be really tricky business. You see, very seldom is it that
two different kinds of polymers will mix together. This doesn't seem to
make sense. Take a look at polyethylene and polypropylene:
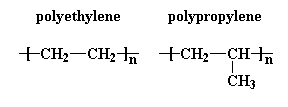
 But they don't. And yes, there is a reason why. It has to do with that old culprit entropy. Entropy is the name we scientists call disorder. This dog is named Entropy. Just say the word "Frisbee" around her and you'll get a good demonstration of what entropy is.
But they don't. And yes, there is a reason why. It has to do with that old culprit entropy. Entropy is the name we scientists call disorder. This dog is named Entropy. Just say the word "Frisbee" around her and you'll get a good demonstration of what entropy is.
This brings us to a little rule we call the second law of thermodynamics. The second law of thermodynamics says that when things change, they will change from a state of order to a state of disorder. Getting things to change in the other direction is very difficult. It's easy to mess up your room, but difficult to clean it up. It's easy to crash a car, but fixing it is much trickier. A change, in your room, in life, in polymers, is more likely to happen if that which is changing changes from a state of more order to a state of less order; that is, if it changes from a state of less entropy to more entropy.
So what does entropy have to do with polymer blends? This will take some explaining. Consider one type of polymer, in the amorphous state. When it's alone, by itself, all its chains are tangled up in each other randomly and chaotically. Entropy runs high in an amorphous polymer.
This presents a problem if you're trying to make polymer blends. You see, one of the biggest reasons two compounds will ever mix together is that they are more disordered mixed together than they are separate. So, mixing is favored by the second law of thermodynamics. But an amorphous polymer is so disordered as it is, that it really doesn't gain that much entropy when it's blended with another polymer. So, mixing is disfavored.
What, then, does this first law of thermodynamics have to do with
blending polymers? This: in order to make two polymers mix, we have to
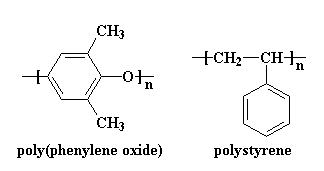
make them have less energy when mixed than they would be separate. Let me use an example to illustrate. Two polymers that do actually mix are
polystyrene and poly(phenylene oxide).
Making Polymers Mix
This presents a challenge to would-be polymer blenders. Without entropy to make polymers blend, how can we ever get two polymers to mix? To make that happen, we have to go back to go back to the first law of thermodynamics. Aha! Just like lawyers, we can use one law to get around another. The first law of thermodynamics says that when things change, they change from a state of more energy to a state of less energy. Think of it this way: it's easier to go to sleep than it is to get out of bed in the morning. Or if you'd like a physics example, a rock on top of a mountain will roll down to the bottom of the mountain more easily than a rock on the bottom will roll to the top. (I learned this the hard way when I was mountain climbing one summer and almost got killed by a falling boulder.)
There are a few other examples of polymer pairs which will blend. Here is a list of a few:
poly(ethylene terephthalate) with poly(butylene terephthalate)Copolymers
poly(methyl methacrylate) with poly(vinylidene fluoride)
But most of the time, the two polymers you want to blend won't be miscible. So you have to play some tricks on them to make them mix. One is to use copolymers. Polystyrene doesn't blend with many polymers, but if we use a copolymer made from styrene and p-(hexafluoro-2-hydroxyisopropyl)styrene, blending is a lot easier.
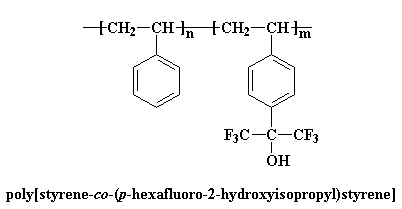
There's another way copolymers can be used to help polymers blend. Let's consider a random copolymer of styrene and acrylonitrile. This copolymer will blend with poly(methyl methacrylate) (PMMA). This is where it gets weird. PMMA won't blend with either polystyrene or polyacrylonitrile.
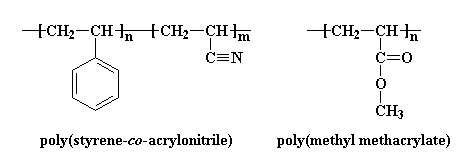
While this method works fine in the laboratory, it could get expensive if you tried to do this industrially. Solvents aren't cheap, and if you're going to evaporate hundreds or thousands of gallons of them, you'll be paying a lot of money. Not to mention the effects on the environment of putting so much of your toxic solvents into the air, or the extra cost of recapturing all that solvent so it could be reused.
So for making blends in large amounts, you heat the two polymers together until you're above the glass transition temperatures of both polymers. At this point they will be nice and gooey, and you can mix them together like a cake mix. This is often done in machines such as extruders. When your material cools, you'll have a nice blend, again, presuming your two polymers are miscible.
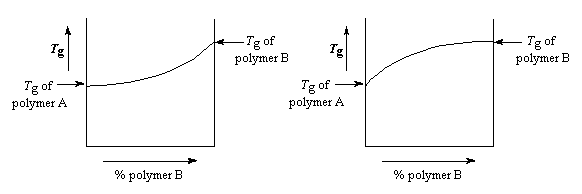
We've been talking about Tgs up until now, but what
holds for Tgs generally holds for other properties.
Mechanical properties, resistance to chemicals, radiation, or heat; they all generally plot the same way as the Tg does with respect to the relative amounts of each polymer in the blend.
This makes altering the properties of your blend fairly simple. When
you vary the amount of the two polymers, you vary the properties. This can be very useful. I'll use the example of poly(phenylene oxide), a.k.a. PPO, to illustrate. PPO is a very heat resistant polymer. This is wonderful. People need heat resistant materials. But it has some drawbacks. It's very hard to process. You see, it's too heat resistant. Amorphous polymers are usually processed by heating them above their Tgs so they get soft and gooey. But with a Tg of 210 oC, heating PPO enough to make it soft and gooey is not only difficult, but expensive.
Enter polystyrene. Remember, polystyrene and PPO blend nicely with each other. Since polystyrene has a
Tg of only about 100 oC, blending polystyrene with PPO drops the Tg of the blend down to temperatures which make the blend much more processable than straight PPO.
Here's a nifty piece of information: NorylTM, the PPO/polystyrene blend that GE sells, uses a special kind of polystyrene, called high-impact polystyrene, or HIPS for short. HIPS is really a mixture of polystyrene and polybutadiene. These two polymers don't blend. The rubbery polybutadiene separates from the polystyrene. But the little
blobs of rubbery polybutadiene make HIPS, and NorylTM, a lot tougher. We call a mixture of two polymers like polystyrene and
polybutadiene that phase separates an immiscible blend.
Immiscible blends aren't really blends at all, because they phase-separate like water and chicken fat in a bowl of homemade chicken soup. But such phase-separated mixtures are also useful, mind you. If you want to read more about them go visit the Immiscible Polymer
Blends Page.
Making Your Own Blends
Blends are usually made in two ways. The first way is to dissolve two
polymers in the same solvent, and then wait for the solvent to evaporate. When the solvent has all gone away, you'll be left with a blend at the bottom of your beaker, presuming your two polymers are miscible.
Properties of Blends
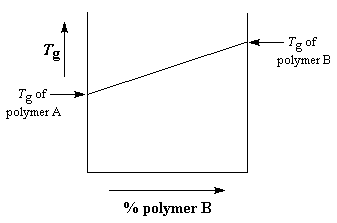
So what are these blends like? How do they behave? In general, a
miscible blend of two polymers is going to have properties somewhere
between those of the two unblended polymers. Take for example the glass transition temperature, or Tg
for short. If we take polymer A and blend it with polymer B, the
Tg will depend on the ratio of polymer A to polymer B in the blend. You can see this in the graph below.

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |