This page is all about polymer crystals. No, this page has nothing to do with polymers as used by the new age community. We're talking about another kind of crystal here. The kind of crystal we're talking about here is any object in which the molecules are arranged in a regular order and pattern. Ice is a crystal. In ice all the water molecules are arranged in a specific manner. So is table salt, sodium chloride. (Oddly, your mother's good crystal drinking glasses are not crystal at all, as glass is an amorphous solid, that is, a solid in which the molecules have no order or arrangement.)
To understand all this talk of crystals and amorphous solids, it helps to go home. Go home? Why? So you can look in your sock drawer, that's why. You see, some people are very neat and orderly. When they put their socks away they fold them and stack them very neatly. Like this:

Other people don't really care about how neat their sock drawers look. Such folk will just throw their socks in the drawer in one big tangled mess. Their sock drawers look like this:

We're going to talk about the neat and orderly crystalline polymers on this page.
So what kind of arrangements do the polymers like to form?
They like to line up all stretched out, kind of like a neat pile of new boards down at the lumber yard.
But they can't always stretch out that straight. In fact, very few polymers can stretch out perfectly straight, and those are ultra-high molecular weight polyethylene, and aramids like Kevlar and Nomex. Most polymers can only stretch out for a short distance before they fold back on themselves. You can see this in the picture.
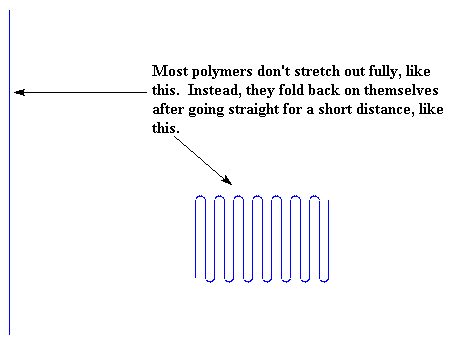
For polyethylene, the length the chains will stretch before they fold is about 100 angstroms.
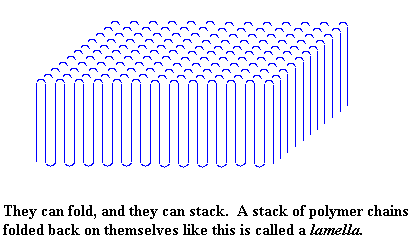
But not only do polymers fold like this. Polymers also form stacks of these folded chains. There is a picture of a stack, called a lamella, right below.
Of course, it isn't always as neat as this. Sometimes part of a chain is included in this crystal, and part of it isn't. When this happens we get the kind of mess you see below. Our lamella is no longer neat and tidy, but sloppy, with chains hanging out of it everywhere!
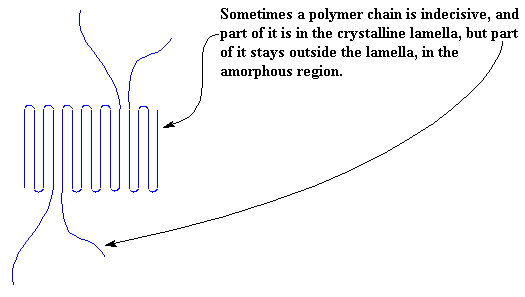
Of course, being indecisive, the polymer chains will often decide they want to come back into the lamella after wandering around outside for awhile. When this happens, we get a picture like this:
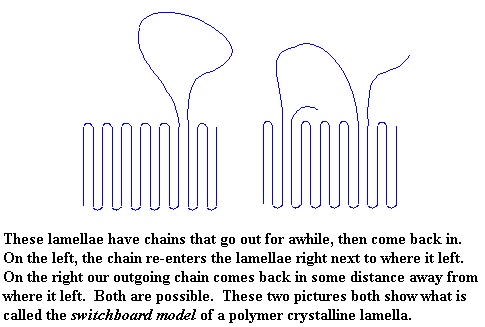
This is the switchboard model of a polymer crystalline lamella. Because we like you, we're going to tell you that when a polymer chain doesn't wander around outside the crystal, but just folds right back in on itself, like we saw in the first pictures, that is called the adjacent re-entry model.
Are you wondering about something? If you look at those pictures up there, you can see that some of the polymer is crystalline, and some is not! Yes folks, most crystalline polymers are not entirely crystalline. The chains, or parts of chains, that aren't in the crystals have no order to the arrangement of their chains. We fancy bigshot scientists say that they are in the amorphous state. So a crystalline polymer really has two components: the crystalline portion and the amorphous portion. The crystalline portion is in the lamellae, and the amorphous potion is outside the lamellae. If we look at a wide-angle picture of what a lamella looks like, we can see how the crystalline and amorphous portions are arranged.
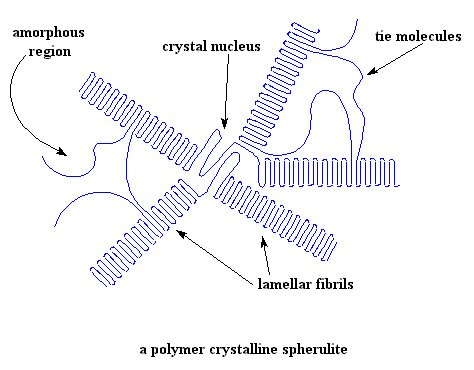
As you can see, lamella grow like the spokes of a bicycle wheel from a central nucleus. (Sometimes we bigshot scientists like to call these spokes "lamellar fibrils".) The fibrils grow out in three dimensions, so they really look more like spheres than wheels. The whole assembly is called a spherulite. In a sample of a crystalline polymer weighing only a few grams, there are many billions of spherulites.
In between the crystalline lamellae, there are regions where there is no order to the arrangement of the polymer chains. These disordered regions are the amorphous regions we were talking about.
As you can also see in the picture, a single polymer chain may be partly in a crystalline lamella, and partly in the amorphous state. Some chains even start in one lamella, cross the amorphous region, and then join another lamella. These chains are called tie molecules.
So you see, no polymer is completely crystalline. If you're making plastics, this is a good thing. Crystallinity makes a material strong, but it also makes it brittle. A completely crystalline polymer would be too brittle to be used as plastic. The amorphous regions give a polymer toughness, that is, the ability to bend without breaking.
But for making fibers, we like our polymers to be as crystalline as possible. This is because a fiber is really a long crystal. Want to know more? Then visit the Fiber Page!
Many polymers are a mix of amorphous and crystalline regions, but some are highly crystalline and some are highly amorphous. Here are some of the polymers that tend toward the extremes:
| Some Highly Crystalline Polymers: | Some Highly Amorphous Polymers: |
| Polypropylene | Poly(methyl methacrylate) |
| Syndiotactic polystyrene | Atactic polystyrene | Nylon | Polycarbonate | Kevlar and Nomex | Polyisoprene | Polyketones | Polybutadiene |
Crystallinity and polymer structure
A polymer's structure affects crystallinity a good deal. If it's regular and orderly, it will pack into crystals easily. If it's not, it won't. It helps to look at polystyrene to understand how this works.
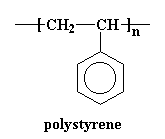
As you can see on the lists above, there are two kinds of polystyrene. There is atactic polystyrene, and there is syndiotactic polystyrene. One is very crystalline, and one is very amorphous.
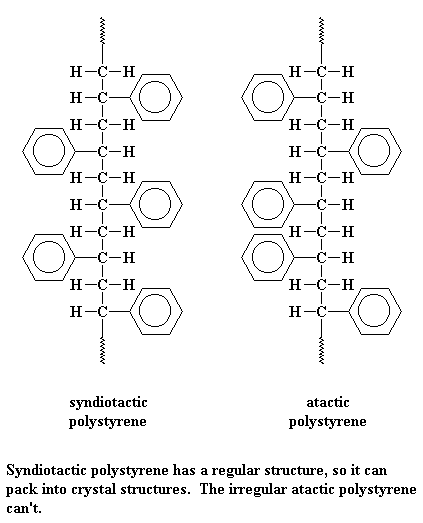
Syndiotactic polystyrene is very orderly, with the phenyl groups falling on alternating sides of the chain. This means it can pack very easily into crystals.
But atactic styrene has no such order. The phenyl groups come on any which side of the chain they please. With no order, the chains can't pack very well. So atactic polystyrene is very amorphous.
Other atactic polymers like poly(methyl methacrylate) and poly(vinyl chloride) are also amorphous. And as you might expect, stereoregular polymers like isotactic polypropylene and polytetrafluoroethylene are highly crystalline.
Polyethylene is another good example. It can be crystalline or amorphous. Linear polyethylene is nearly 100% crystalline. But the branched stuff just can't pack the way the linear stuff can, so it's highly amorphous.

Polyesters are another example. Let's look at the polyester we call poly(ethylene terephthalate).
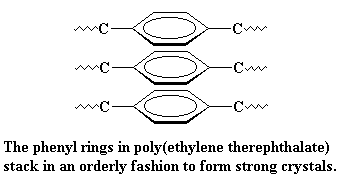
The polar ester groups make for strong crystals. In addition, the aromatic rings like to stack together in an orderly fashion, making the crystal even stronger.
Crystallinity and intermolecular forces
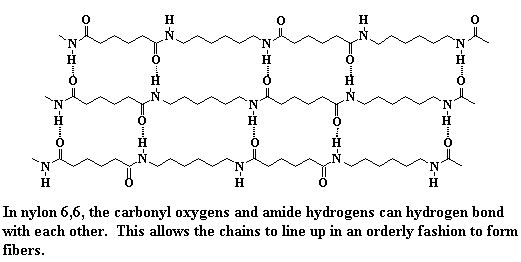
Intermolecular forces can be a big help for a polymer if it wants to form crystals. A good example is nylon. You can see from the picture that the polar amide groups in the backbone chain of nylon(6,6) are strongly attracted to each other. They form strong hydrogen bonds. This strong binding holds crystals together.
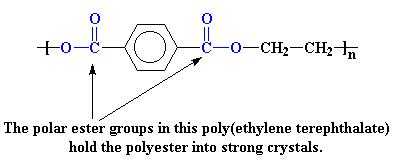
How Much Crystallinity?
Remember we said that many polymers contain lots of crystalline material and lots of amorphous material. There's a way we can find out how much of a polymer sample is amorphous and how much is crystalline. This method has its own page, and it's called differential scanning calorimetry.

|
Return to Level Three Directory |

|
Return to Macrogalleria Directory |