
There's more here than high weights. Metallocene polymerization is also good for making polymers of very specific tacticities. It can be tuned to make isotactic and syndiotactic polymers, depending on what you need.
I knew I couldn't dodge this question forever. I could say simply, metallocene polymerization is polymerization catalyzed by metallocenes.
I figured you'd want to know that. Again, I could give simple answer that a metallocene is a positively charged metal ion sandwiched between two negatively charged cyclopentadienyl anions.
My what an inquisitive mind you have! And your inquisitiveness will not go unrewarded! I will tell you that a cyclopentadienyl anion is a nifty little ion that's made from a little molecule called cyclopentadiene. I'm guessing you're just about to ask what that is, so I put a little picture of it right down below:
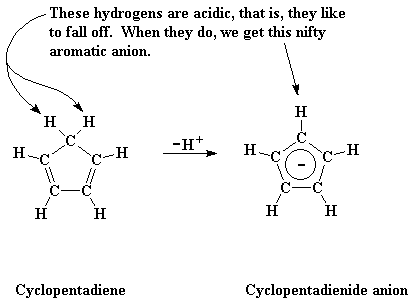
You may notice that most of the carbon atoms have one hydrogen, but one carbon atom has two hydrogens. One of those two hydrogens are acidic, that is, one will fall off very easily. When this happens, it leaves its bonding electrons (that is, an electron pair) behind. So the carbon it left now has only one hydrogen, just like the others, plus an extra pair of electrons.
Don't you just hate it when you've got extra electrons and nothing to do with them?
But this is not the case with cyclopentadiene, fear not! See those two double bonds in the molecule? Each of those has two electrons, remember, making four in all. Add those two extra electrons on the carbon that lost the hydrogen, and we have six.
This is important. Six electrons in a ring molecule like this will make the ring aromatic. If you've had enough organic chemistry to know what this means, great! If you haven't just know that it means the ring in this anionic form will be very stable.
Got that?
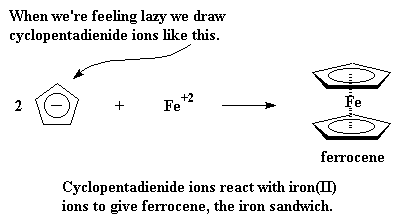
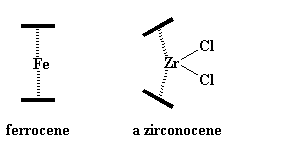
These cyclopentadienide ions have a charge of -1, so when a cation comes along, like Fe with a +2 charge, two of the anions will form an iron sandwich. That iron sandwich is called ferrocene.
Sometimes a metal with a bigger charge is involved, like zirconium with a +4 charge. To balance the charge, the zirconium will bond to two chloride ions, -1 charge on each, to give a neutral compound.
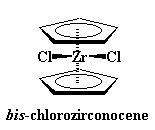
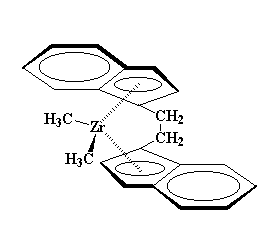
Zirconocenes are a little different from ferrocene. You see, those extra ligands, the chlorines, take up space. It's hard for them to squeeze in-between the cyclopentadienyl rings. So to make room for the chlorines, the rings become tilted with respect to each other, opening like a clam shell. This gives the chlorines space to breathe. Take look at the picture showing this tilt:
We can use some derivatives of bis-chlorozirconocene to make polymers. Take this one for example:
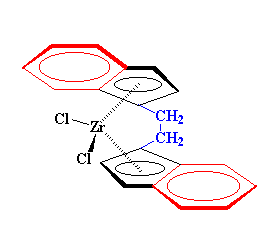
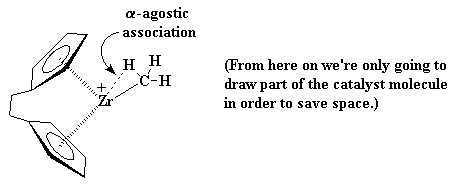
Learn about alkene-metal complexation
Skip to the polymerization
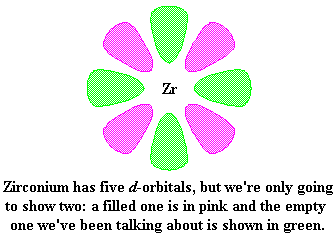
Let's look again at zirconium for a moment. This picture shows zirconium and two of its d-orbitals. Now to be sure, zirconium has five d orbitals, but we're only going to show two right now for clarity.
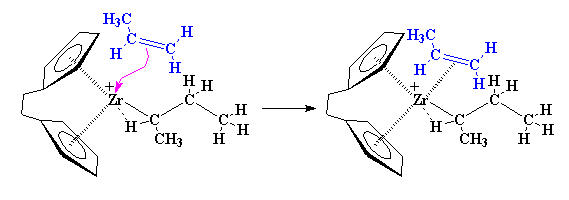
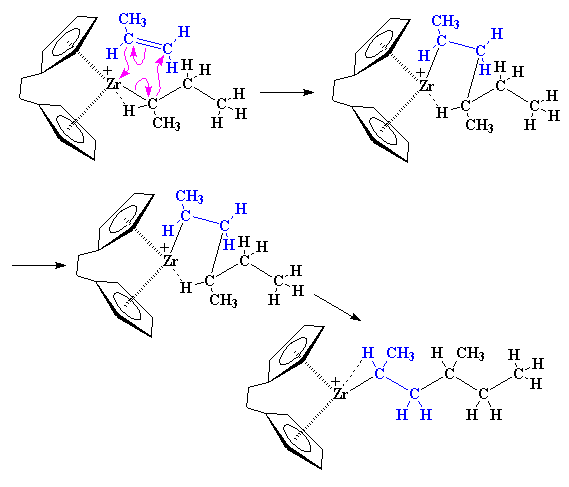
Being back where we started, another propylene monomer can come along and react just like the first one did.
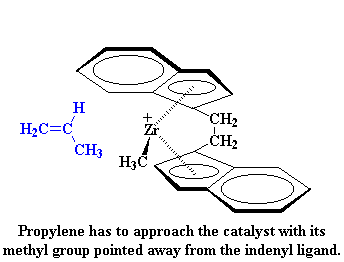
So why do we get an isotactic polymer? Let's look at the catalyst and an incoming propylene monomer for a minute. As you can see, the propylene monomer always approaches the catalyst with its methyl group pointed away from the indenyl ligand.
When the second monomer is added, it has to approach from the other side, and it also has to point its methyl group away from indenyl ring:
Ok folks, this brings up a question. Knowing why this catalyst gives isotactic polypropylene, what kind of catalyst would give syndiotactic polypropylene?
Have you figured it out yet? It's a catalyst such as this bad boy, which was investigated by Ewen and Asanuma.
So what does this mean for our polymerization? It means something really strange will happen. When the zirconocene is in the rac form, poly propylene monomer can only approach in an orientation which will give isotactic polypropylene.
Alkene-metal complexes
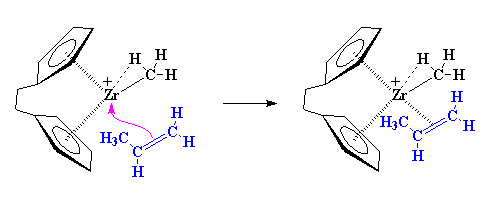
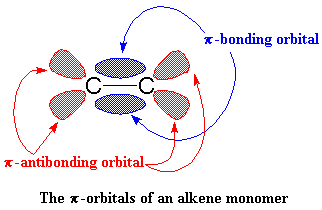
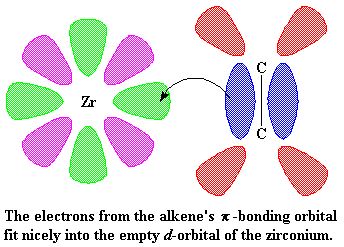
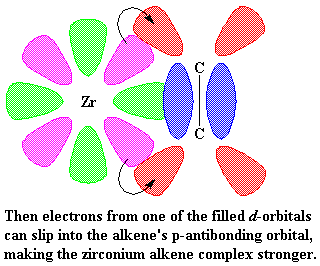
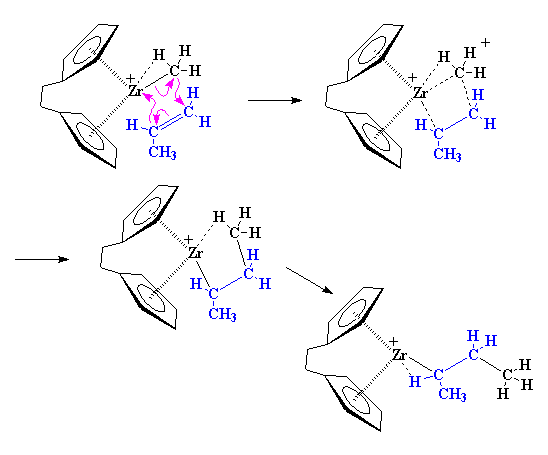
This is where it starts to get interesting. Suppose at this point that a vinyl monomer showed up, let's say, a molecule of propylene. The zirconium is going to enjoy this. To understand why, let's take a look at vinyl monomer, specifically, its double bond. A carbon-carbon double bond, is made up of a σ bond and a π bond. We're going to take a closer look at that
π bond.
The Polymerization
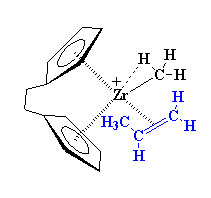
The precise nature of the complex between the zirconium and the propylene is complicated. So to make things simple we're going to just draw it like we did earlier from now on, like this:
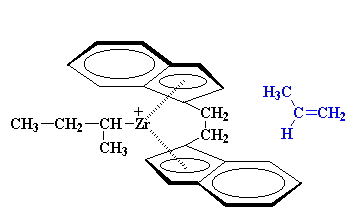
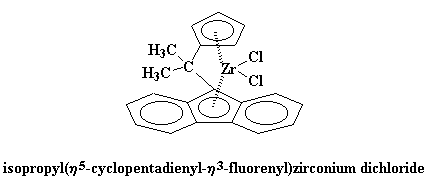
Identity Crisis
But metallocene catalysts can do even stranger things than that. Let's consider bis(2-phenylindenyl)zirconium dichloride. This metallocene, as you see below, has no bridge between the two indenyl rings.
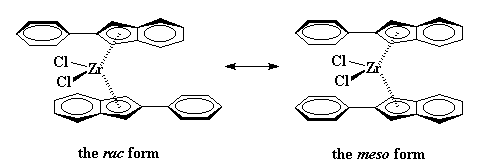
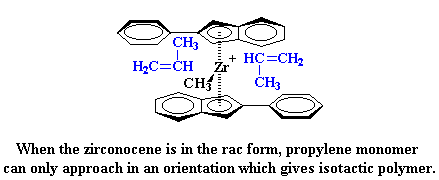
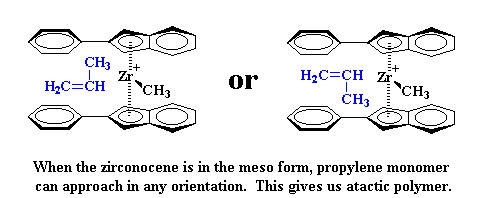

|
Return to Level Four Directory |

|
Return to Macrogalleria Directory |