So then how does it work? Something like this: Take your Ziegler-Natta catalyst, usually TiCl3 or TiCl4, along with an aluminum based co-catalyst, and place in the monomer at midnight on the night of the full moon. Then place the beaker on the ground in a circle of lighted candles, and then write the word "isotactic" or "syndiotactic", depending of the tacticity you desire, in runic letters on the side of the beaker with the blood of a freshly slain goat. The goat must be less than one year old, and without blemish. Then one must recite aloud the Ziegler-Natta incantation seven times, followed by the tacticity dance. If the polymerization is successful, a cold and violent wind will quickly arise and extinguish the candles, and then die away as quickly as it arose. It is important that one fast for three days before and after carrying out the ceremony. Following this little procedure usually does the trick.
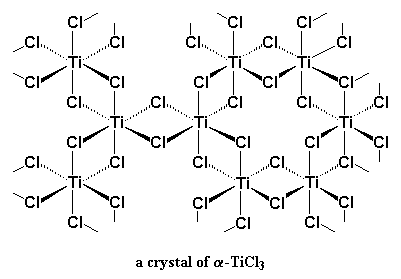
Ok, so that's not really how it works, but our knowledge of how Ziegler-Natta polymerization works, and why one initiator system will work better than another is rather limited. Picking the right conditions to make a Ziegler-Natta polymerization work often feels more like magic than science. But we do know a little bit. We know that it involves transition metal catalyst, like TiCl3. We also know that co-catalysts are involved, and these are usually based on group III metals like aluminum. Most of the time our catalyst/co-catalyst pair are TiCl3 and Al(C2H5)2Cl, or TiCl4 with Al(C2H5)3.
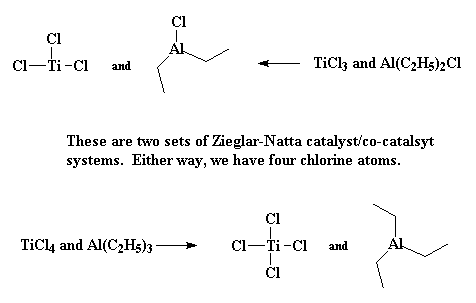
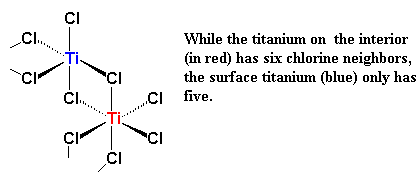
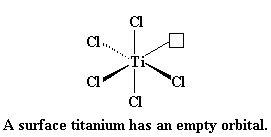
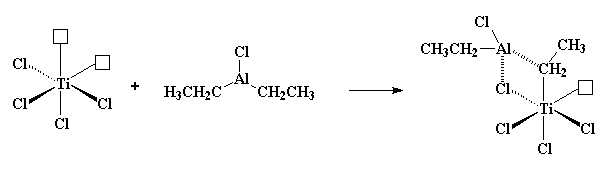
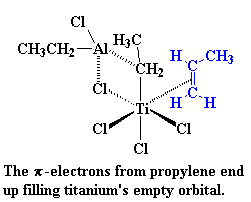
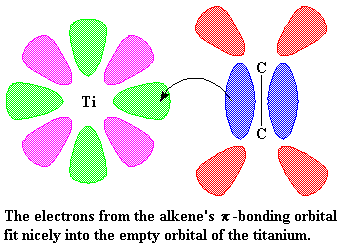
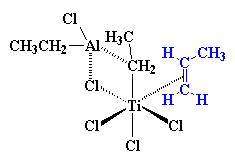
So then a vinyl monomer like propylene comes along. There are two electrons in the π-system of a carbon-carbon double bond. Those electrons can be used to fill the empty orbital of the titanium. We say that the propylene and the titanium form a complex, and we draw it like this:
Learn about alkene-metal complexation
Skip to the polymerization
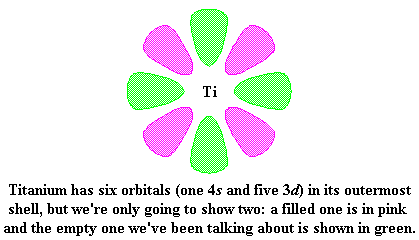
Let's look again at titanium for a moment. This picture shows titanium and two of its outermost orbitals. (Yes, it has more than two, but we're only going to show two right now for clarity.)
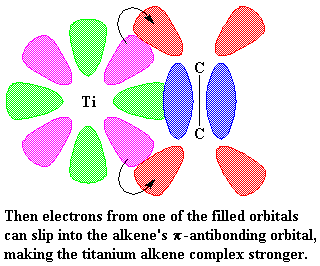
The precise nature of the complex between the titanium and the propylene
is complicated. So to make things simple we're going to just draw it like
we
did
earlier from now on, like this:
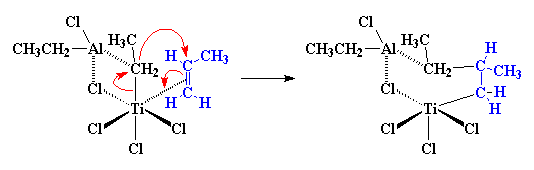
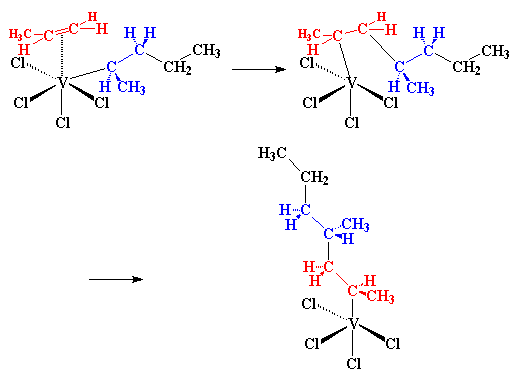
What happens next is what we call a migration. We don't know why
this happens, we just know it happens. But the atoms rearrange themselves
to form a slightly different structure, like this:
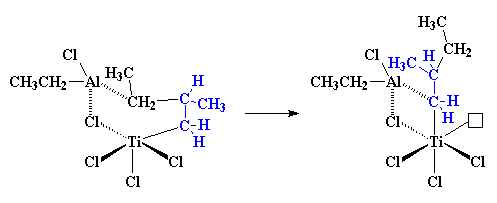
So when another propylene molecule comes along, the whole process starts
all over, and the end result is something like this:
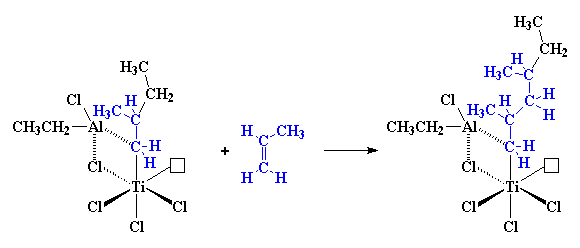
and of course, more and more propylene molecules react, and our polymer
chain grows and grows. Take a look at the picture, and you'll see that
all the methyl groups on the growing polymer are on the same side of the
chain. With this mechanism we get isotactic polypropylene. For some
reason, the incoming propylene molecule can only react if it's pointed in
the right direction, the direction that gives isotactic polypropylene.
We're not sure why this happens, we just know that it happens.
If you want to see a movie of isotactic Ziegler-Natta polymerization,
click
here!
Part Two: Syndiotactic Polymerization
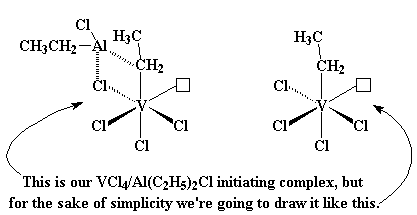
The catalyst system we just looked at gives isotactic polymers. But other
systems can give syndiotactic polymers. The one we're going to look at is
based on vanadium rather than titanium. That system is
VCl4/Al(C2H5)2Cl. It looks
like the picture you see on the left, not too different from the titanium
system we just looked at. But to simplify things, during this little
discussion we're going to just draw what you see on the right.
If you'd like to see a movie of how syndiotactic Ziegler-Natta
polymerization takes place, click here!
Alkene-metal complexes
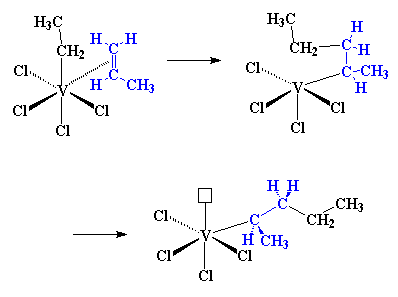
This is where it starts to get interesting. Suppose at this point that a
vinyl monomer showed up, let's say, a molecule of propylene. The titanium
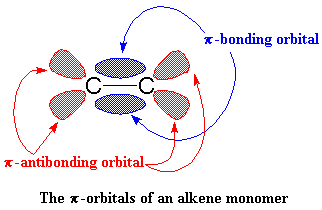
is going to enjoy this. To understand why, let's take a look at vinyl
monomer, specifically, its double bond. A carbon-carbon double bond, is
made up of a σ bond and a π bond. We're going to take a closer look at that
π bond.
The Polymerization
Part One: Isotactic Polymerization
Limitations
Ziegler-Natta polymerization is a great way to make polymers from
hydrocarbon monomers like ethylene and propylene. But it doesn't work of
for some other kinds of monomers. For example, we can't make poly(vinyl chloride) by Ziegler-Natta polymerization.
When the catalyst and cocatalyst come together to form the initiating
complex, radicals are produced during intermediate steps of the reaction.
These can initiate free radical polymerization
of the vinyl chloride monomer. Acrylates are
out, too, because Ziegler-Natta catalysts often initiate anionic vinyl polymerization in those monomers.
Moving Forward
For a long time, Ziegler-Natta polymerization was the most useful and
versatile reaction for producing polymers of a specific desired tacticity.
But recently a new type of polymerization, also using metal complexes as
initiators, has been developed, called metallocene
catalysis polymerization. It's hot, so go read about it!

|
Return to Level Four Directory |

|
Return to Macrogalleria Directory |