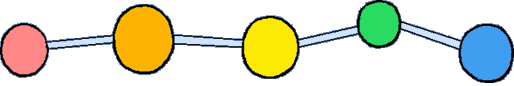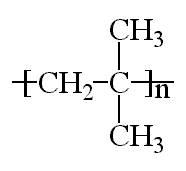
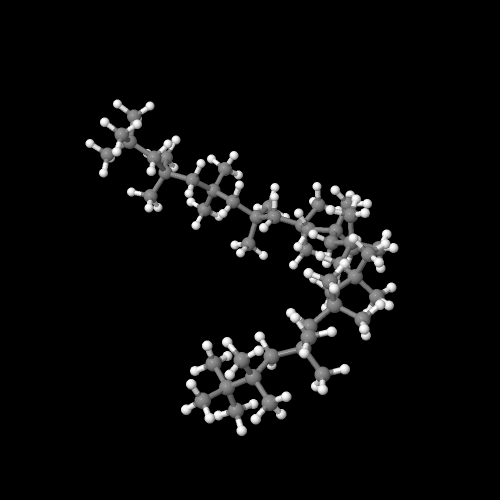
The model on the right above is an image of the pdb model you can view by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up with the 3D model in it when you are ready to come back here.
Polyisobutylene, sometimes called butyl rubber, and other times PIB, is a vinyl polymer. It's very similar to polyethylene and polypropylene in structure, except that every other carbon is substituted with two methyl groups. It is made from the monomer isobutylene, by cationic vinyl polymerization.
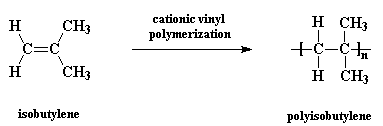
And this is that monomer isobutylene:
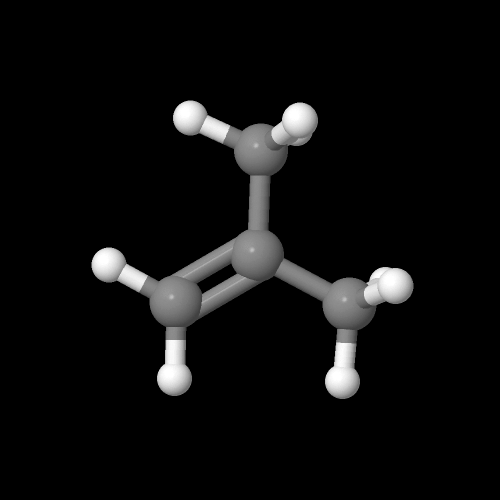
The model above is an image of the pdb model you can view by clicking here or you can just click on the image itself.
Either way, be sure to close the new window that opens up with the 3D model in it when you are ready to come back here.
Usually, a small amount of isoprene is added to the isobutylene. The polymerization is carried out at a right frosty -100 oC, or -148 oF for you Americans out there. This is because the reaction is so fast we can't control it unless we freeze it colder than a brass toilet seat in the Yukon.
Polyisobutylene was first developed during the early 1940s. At that time, the most widely used rubber was natural rubber, polyisoprene. Polyisoprene was an excellent elastomer, and easy to isolate from the sap of the hevea tree. Huge plantations thrived in Malaysia and grew hevea trees to supply the world's rubber needs. There was only one slight problem, and that was that Malaysia had just been conquered by the Imperial Japanese Army, and wouldn't you know we just so happened to be fighting the Second World War against them right at that moment. Before the war was over more than sixty million people would be dead. Deprived of natural rubber, the Allied nations did some quick thinking and came up with PIB. It obviously worked, because the Allies won the war.
Ok, we didn't actually invent polyisobutylene during the war. It had been invented long before the war by chemists in Germany. There's irony! But it wasn't very useful until American chemists came up with a way to crosslink it. What they did was to copolymerize isobutylene with a little bit, say, around one percent, isoprene. This is isoprene:
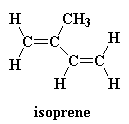
When isoprene is polymerized with the isobutylene we get a polymer that looks like this:
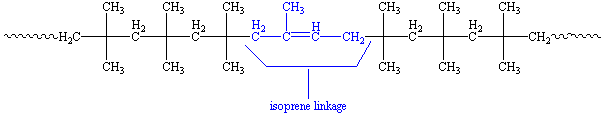
About one or two out of every hundred repeat units is an isoprene unit, shown in blue. These have double bonds, which means the polymer can be crosslinked by vulcanization just like natural rubber. What is this vulcanization? To find out, click here.
Other polymers can be used as rubber, too. Here are some:

|
Return to Level Two Directory |

|
Return to Macrogalleria Directory |